MARIA BELÉN ZANCHETTA1, 2, RUBÉN ABDALA1, 2, FABIO MASSARI1, 2, PAULA REY1, 2, RODOLFO SPIVACOW1, LARA MIECHI1, VANESA LONGOBARDI1, 2, LUCAS R. BRUN3, 4
1 Instituto de Diagnóstico e Investigaciones Metabólicas (IDIM), Buenos Aires, 2Cátedra de Osteología y Metabolismo Mineral, Universidad del Salvador, Buenos Aires, 3Laboratorio de Biología Ósea, Universidad Nacional de Rosario, Santa Fe, 4Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina
Resumen Recientemente el grupo de trabajo europeo sobre sarcopenia en adultos mayores (EWGSOP2) recomendó nuevos criterios y valores de referencia para el diagnóstico de sarcopenia. El objetivo del presente trabajo fue evaluar la prevalencia de sarcopenia en mujeres postmenopáusicas en nuestro medio y su relación con densidad mineral ósea, caídas y fracturas por fragilidad. Este es un estudio de diseño transversal en el cual se incluyeron un total de 250 mujeres ambulatorias mayores de 60 años. La densidad mineral ósea (DMO) de columna lumbar y cadera y la composición corporal fueron evaluados por absorciometría dual de rayos X (DXA). La fuerza fue evaluada por dinamometría de puño; para el rendimiento físico se utilizó caminata de 4 m y la prueba de levantarse y sentarse de una silla (5 repeticiones). La sarcopenia se definió de acuerdo a EWGSOP2 como baja fuerza muscular (dinamometría) y baja masa muscular (índice de masa muscular esquelética por DXA). El 4% de las mujeres cumplía con los criterios de sarcopenia siendo aún mayor en aquellas ≥ 80 años. Las mujeres con sarcopenia presentaron significativamente mayor frecuencia de caídas, osteoporosis y fracturas vertebrales. El riesgo de fracturas por fragilidad se vio incrementado 6 veces en las mujeres con sarcopenia. El diagnóstico de sarcopenia podría considerarse una herramienta útil para identificar a aquellos adultos con riesgo incrementado de caídas y fracturas.
Palabras clave: sarcopenia, fuerza muscular, rendimiento físico, fracturas, caídas
Abstract Recently, a new consensus of the European Working Group on Sarcopenia in Older People (EWSOP2) recommended new cut-off points for the diagnosis of sarcopenia. The aim of the present manuscript was to assess the prevalence of sarcopenia in postmenopausal women and its relationship with bone mineral density, falls and fragility fractures according to EWGSOP2. In this cross-sectional study, 250 ambulatory postmenopausal women over 60 years of age were included. Lumbar spine and hip bone mineral density (BMD) and whole-body composition were assessed by dual-energy X-ray absorptiometry (DXA). Muscle strength was evaluated by handgrip dynamometry and physical performance by a 4-m walk gait speed and five-repetition sit-to-stand test. Sarcopenia was defined according to EWGSOP2 as low muscle strength (handgrip) and low muscle mass (appendicular skeletal muscle mass index by DXA). A sarcopenia prevalence of 4% was found in the whole group increasing with age being 12.5% in ≥ 80- year-old. A higher percentage of falls, prevalence of osteoporosis and vertebral fractures were found in the sarcopenic group. Sarcopenia increased 6.0-fold the likelihood of having a fragility fracture. Women with sarcopenia had significantly lower femoral neck BMD and higher frequency of falls and vertebral fractures. According to our results, identifying patients with sarcopenia might be a useful tool to detect adults at higher risk of falls and fractures.
Key words: sarcopenia, muscle strength, physical performance, fractures, falls
Dirección postal: Rubén Abdala, Instituto de Diagnóstico e Investigaciones Metabólicas (IDIM), Libertad 836, 1012 Buenos Aires, Argentina
e-mail: rubenabdala92@outook.com
Sarcopenia is a progressive and generalized skeletal muscle disorder that is associated with an increased likelihood of adverse outcomes including falls, fractures, physical disability and poor quality of life1. It is prevalent in older populations and is widely considered one of the major causes of disability in older people. Furthermore, a higher risk of death from all causes compared with nonsarcopenic subjects was found in > 60 years old (NANHES III HR: 1.29) and mainly in > 80 years old (Aging and Longevity Study, HR: 2.32)2, 3.
The onset of sarcopenia has been related to several factors as aging, malnutrition, muscle disuse or sedentary lifestyle, chronic inflammatory or endocrine disease, neurodegenerative diseases, cachexia, fat infiltration, and certain drug treatments4, 5.
In recent years, various international consensuses have brought these fundamental concepts to the clinical practice. The European Working Group on Sarcopenia in Older People (EWGSOP) developed a practical clinical definition and consensus diagnostic criteria for age-related sarcopenia, recommending the use of the presence of both low muscle mass and low muscle function (strength or performance) for diagnosis4. Recently, a new consensus (EWGSOP2) recommended low muscle strength as the primary parameter to assess sarcopenia and new cut-off points to increase harmonization of sarcopenia studies1.
According to EWGSOP, the prevalence of sarcopenia was found from 1 to 29% for older adults living in the community and 14-33% for those living in long-term care institutions6. Prevalence of sarcopenia in 60-70-year-old is reported as 5-13%, while the prevalence ranges from 11 to 50% in people > 80 years7. A study of the Foundation for the National Institutes of Health with 4984 patients older than 60 years found that the average age of patients with sarcopenia was 70.5 years in men and 71.6 years in women8. Furthermore, most studies reported that there is no significant association with sarcopenia prevalence according to gender6.
In addition, a higher risk of falls should be expected in sarcopenia due to the main loss of fast-switch type II fibers and loss of motor-neurons9, 10. A prospective study with a 2-year follow-up showed that patients with sarcopenia had a higher risk of falls than non-sarcopenic patients (27.3% vs. 9.8%; p < 0.001)11. Moreover, it was found that after 4 years of intervention with physical activity and dietary supplement, sarcopenic women older than 75 years had a lower incidence of falls than the control group (23% vs. 29%) due to prevention of significant declines in lower extremity muscle mass, strength and mobility12. Therefore, the relationship between fractures and sarcopenia should also be expected. Fall-related fractures are one of the most serious consequences and hip fracture is a major complication affecting osteoporotic and sarcopenic elderly people. In a recent review where eight studies were evaluated, it was suggested that sarcopenia could be a predictor of risk for hip fracture10. Working in the osteoporosis field, we intend to decrease fracture risk in patients; but this goal will never be completely possible if we do not integrate muscle assessment into our routine practice.
The aim of our study was to assess the prevalence of sarcopenia in a group of postmenopausal women older than 60 years of age and its relationship with bone mineral density (BMD), falls and fragility fractures. We hypothesized that sarcopenic postmenopausal women would have a higher prevalence of osteoporosis, falls and fractures.
Materials and methods
Postmenopausal Caucasian women over 60 years of age, who visited our bone clinic, were invited to have their muscle health assessed. In this cross-sectional study, 250 consecutive patients were assessed. Potential causes of secondary sarcopenia (cancer, cardiac and pulmonary disease, among others) were considered as exclusion criteria.
Weight (kg) and height (m) as anthropometric parameters were recorded to calculate body mass index (BMI = weight/ height2 [kg/m2]). Clinical antecedents and risk factors for osteoporosis were recorded, as well as fragility fractures and fall history were specifically assessed. Wrist, hip and vertebrae were the fragility fractures considered. For clinical fractures, confirmation X-rays were taken into account. For falls the WHO definition, as the event of suddenly go down onto or towards the ground unintentionally or accidentally from one´s height was considered. We took into account at least two or more falls occurred in the last twelve months. Physical activity was considered as hours in a week devoted to gymnastics or any kind of sport. BMD and osteoporosis diagnosis by dual-energy X-ray absorptiometry (DXA), and vitamin D levels were also considered.
Sarcopenia was confirmed, according to the EWGSOP2, as low muscle strength plus low muscle quantity. If low physical performance was also found, sarcopenia was considered severe1.
The study was conducted according to the Declaration of Helsinki and was approved by a local ethics committee.
Written informed consent was obtained from all participants.
The BMD (g/cm2) was measured by DXA with GE Lunar Prodigy equipment (GE Lunar, Madison, WI, USA) at the lumbar spine (L1-L4), femoral neck and total hip. The coefficient of variation was less than 1%. According to WHO, a T-score ≤ -2.5 at the hip or lumbar spine was considered osteoporosis and a T-score between -1 and -2.5 was classified as low BMD13. The whole-body composition was performed using the specific software provided by the manufacturer.
Briefly, using specific anatomic landmarks, legs, arms, and trunk were isolated on the skeletal X-ray anterior view by DXA. Whole-body scans provided measurements of total and regional lean mass (kg), fat mass (kg) and bone mineral content (kg). Muscle mass was determined in the four limbs by a DXA scan as appendicular skeletal muscle mass (ASM) and defined an appendicular skeletal muscle mass index as ASM/height2 (kg/m2)13. According to the EWGSOP2, a limit of 5.5 kg/m2 or 15 kg for ASM in women was considered to be the gender-specific cut-off for low muscle mass1. Both, BMD and whole-body composition scans, were performed by the same qualified technician.
Muscle strength and physical performance measurements were performed by the same trained physician. Muscle strength was evaluated by hand-grip strength assessment (Baseline Hydraulic Hand Dynamometer, USA) in the dominant hand. Hand-grip strength is strongly correlated with age and low muscle mass and also correlates with leg strength14. The best value of three determinations was taken into account.
According to the EWGSOP2, 16 kg was considered the cutoff in women1. Physical performance was evaluated with a 4-m walk gait speed and the five-repetition sit-to-stand test.
The 4-m walk gait speed was performed after a practice test and low walking speed was defined as walking slower than 0.8 m/s. Participants were instructed to stand with both feet touching the starting line and to begin walking at their usual pace. In the sit-to-stand test, the time of five repetitions without hand help was recorded. Low sit-to-stand test was defined as > 15 seconds1, 15.
Data are expressed as mean±SD and the Shapiro-Wilk and Bartlett tests were used to assess normality and equal variances respectively. The Student’s t-test or Mann-Whitney test was used as appropriate to compare results between groups. Correlations were analyzed with Pearson or Spearman correlation tests as appropriate. A multivariate logistic regression odel was used to adjust if there were possible confounding factors. Differences were considered significant if p < 0.05. Statistical analyses were performed with STATISTIX 7.0 Copyright©1995, 2000 Analytical software (Statsoft).
Results
This study included 250 postmenopausal women over 60 y (70.4 ± 7.7 years old). A prevalence of 4% (n = 10) of sarcopenia was found in the whole group. As expected, a significant difference in age was noticed between sarcopenia and the non-sarcopenia groups. In addition, women with sarcopenia had significantly lower weight and height compared to non-sarcopenia group. However, no differences in BMI were observed (Table 1).
A significant difference in the prevalence of sarcopenia was found according to age (c2 p<0.0142): 1.4% in the 60-69 group, 4.9% in the 70-79 and 12.5% in the ≥ 80- year group.
Muscle assessments (muscle mass, hand-grip strength and gait speed) were significantly different between sarcopenia and non-sarcopenia groups (Table 2). Total lean appendicular mass and lean mass in arms and legs were found to be significantly lower in sarcopenic subjects; but the appendicular skeletal muscle mass index was not found to be significantly different.
Femoral neck BMD was significantly lower in postmenopausal women with sarcopenia compared to nonsarcopenic women. However, no differences between groups were found in lumbar spine and total hip BMD (Table 3).
In addition, a higher number of vertebral fractures was found in the sarcopenic group compared to nonsarcopenic women (60% vs. 7.1%, OR 6.0, IC95%: 2.5- 24.2; Fisher’s exact test p = 0.0012). Despite a higher frequency of osteoporosis and of hip and wrist fractures in the sarcopenia group, no significant differences were observed (Table 4). Furthermore, the sarcopenic postmenopausal women had a significantly higher frequency of falls in the previous year. No differences in the percentage of total body fat, 25(OH) vitamin D levels, and physical activity were observed. Moreover, we analyzed each criterion and its association with falls and we found that low muscle strength was associated with recurrent falls in the last year (OR: 3.8, 95%CI: 1.24-11.96, p = 0.02) (Table 4).

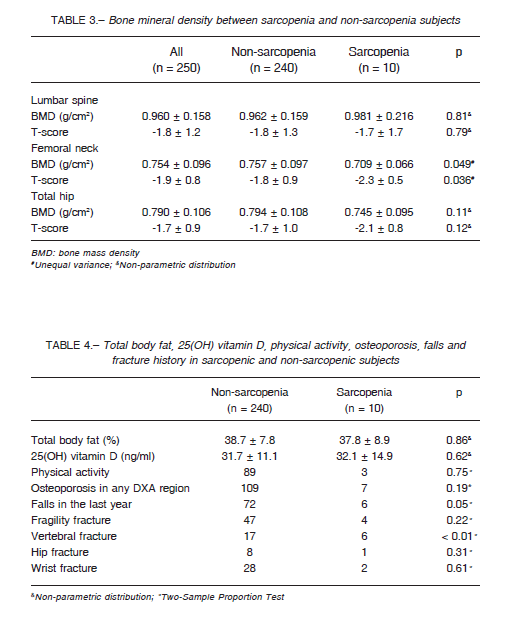
In Table 5 we compared fractured vs non-fractured subjects. Significantly lower muscle mass in legs, handgrip strength, physical performance, and femoral neck and total hip BMD were observed in those who had a previous fracture (p < 0.01) (Table 5).
Finally, we performed logistic regression analysis with fragility fracture as a dependent variable and each sarcopenia component as an independent variable through multivariate logistic regression. Those individuals who had better physical performance (gait speed) showed a decreased risk for fracture (OR 0.57, 95% CI: 0.41-0.78, p < 0.01).
Discussion
We found a prevalence of sarcopenia of 4% in this group of ambulatory postmenopausal Caucasian women over 60 years of age. Sarcopenic women had a significantly higher vertebral fracture history, even adjusted for age, BMI and BMD. Consistently, they also had lower muscle strength and physical performance and fall history. The low muscle strength was associated with recurrent falls in the last year (OR: 3.8). The sarcopenia prevalence found is similar to that of previous reports that showed up to 29% in older living-community adults6. Furthermore, we found that the prevalence of sarcopenia increased with age, being 12.5% in ≥ 80- year-old according to previous reports16, 17. Beyond the age of 50, loss of leg muscle mass (1-2% per year) and loss of strength (1.5-5% per year) have been reported18. In addition, muscle mass and physical performance, have an inverse relationship with age. Patel et al., using the definition of the first EWGSOP consensus, determined that the loss of muscle mass was 30-50% between 40 and 80 years of age and that there was an up to 3% yearly deterioration in physical performance after age 6019.
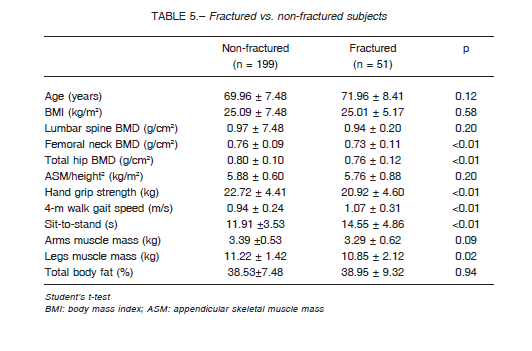
It has been suggested that sarcopenia may contribute to an increase in the risk of fractures associated with aging and low BMD. We found that sarcopenic women had significantly lower femoral neck BMD and higher falls in the previous year (60% vs. 30%). Accordingly, we found a higher prevalence of vertebral fracture in the sarcopenic group (60% vs. 7.1%) and therefore sarcopenia increased 6.0-fold the likelihood of having had a vertebral fragility fracture. Despite higher frequency of fragility fractures and higher frequency of osteoporosis in sarcopenia group compared to non-sarcopenia women, no statistical differences were found. Perhaps the low prevalence of sarcopenia in this cohort does not allow to reach statistical differences.
The relationship between sarcopenia and wrist and hip fractures may seem clear because these fractures are preceded by a fall. However, its relationship with vertebral fractures has not been reported extensively. In a previous report of 67 women with vertebral fractures, the prevalence of sarcopenia was 66.6% and the presence of more than two vertebral fragility fractures represented an increased risk of sarcopenia (OR 2.63)20. The relationship between sarcopenia and vertebral fractures might be attributed to an intimate bone and muscle intercommunication. Indeed, recent studies indicate a role for neuronal regulation of not only muscle but also bone metabolism, bone signaling pathways like receptor activator of nuclear factor kappa- B ligand (RANKL) implicated in muscle biology, myokines affecting bone and possible bone-to-muscle communication21.
Previous studies showed that women with low BMD, with or without sarcopenia, had a higher risk of fracture than women with only sarcopenia and normal subjects.
However, in subjects with sarcopenia without low BMD, no increase in fracture risk was observed22, 23. A recent study suggested that muscle mass adjusted for height (ASM/ height2) appears to be a relevant risk factor for osteoporosis in postmenopausal women24. However, even though we found significant differences in lean appendicular mass (total, arms and legs) between sarcopenic and non-sarcopenic women, no differences in ASM/height2 was observed in this study because both parameters decreased proportionally.
In addition, the prevalence of sarcopenia in women with hip fracture was found to be between 37 and 58%25,26.
Landi et al reported that sarcopenic participants were over three times more likely to fall during a follow-up period of 2 years relative to non-sarcopenic individuals27. In a cohort of women with hip fractures, a significant positive correlation between ASM/height2 and BMD at both total proximal femur and femoral neck was described27. Furthermore, 58% were diagnosed with sarcopenia and 74% had osteoporosis, revealing a high prevalence of these two conditions in fractured women. Besides, lean mass and appendicular muscle mass indexes were associated with the risk of fracture in postmenopausal women independently of BMD and clinical risk factors28. On the other hand, another study did not show any relationship between sarcopenia and femur BMD, recurrent falls or hip fractures29. However, this lack of association might be attributed to the use of bioelectrical impedance analysis (BIA) instead of DXA for measuring muscle mass.
As expected, lower muscle mass, hand-grip strength, physical performance, and hip BMD were observed in subjects with a previous fracture. In addition, a higher physical performance (gait speed) showed a decreased risk for fracture (OR 0.57).
In summary, sarcopenia was associated with low handgrip strength, gait speed and sit-to-stand performance showing a decreased muscle quality. However, no differences in appendicular skeletal muscle mass index were found, being consistent with the EWGSOP2, which also use the ASM (15 kg) as criteria.
Some limitations of this study should be pointed out: the cross-sectional study design, the small sample, the potential selection bias and the recall bias related to fall history. Longitudinal studies are necessary to better determine cause and effect.
In conclusion, according to the new EWGSOP2 consensus, the prevalence of sarcopenia in our group of postmenopausal women over 60 was 4% reaching 12.5% in older women (≥ 80 y). Sarcopenic postmenopausal women over 60 had lower muscle strength, physical performance and femoral neck BMD and higher prevalence of falls and vertebral fractures increasing 6.0-fold the likelihood of having a fragility fracture.
Our data emphasizes the importance of the evaluation of muscle mass and function, particularly in subjects with low BMD or osteoporosis, who would have a higher risk of fractures. In addition, the bone-muscle unit should be considered as a whole, and sarcopenia, fall risk and bone quality must be addressed simultaneously, especially in the very elderly. Working in the osteoporosis field, we intend to decrease patients’ fracture risk; but this goal will never be fully attained if we do not integrate muscle assessment into our routine practice.
Conflict of interests: None to declare
References
1. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48: 16-31.
2. Brown JC, Harhay MO, Harhay MN. Sarcopenia and mortality among a population-based sample of communitydwelling older adults. J Cachexia Sarcopenia Muscle 2016; 7: 290-8.
3. Landi F, Cruz-Jentoft AJ, Liperoti R, et al. Sarcopenia and mortality risk in frail older persons aged 80 years and older: results from ilSIRENTE study. Age Ageing 2013; 42:203-9.
4. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39: 412-23.
5. Sayer AA, Syddall H, Martin H, Patel H, Baylis D, Cooper C. The developmental origins of sarcopenia. J Nutr Health Aging 2008; 12: 427-32.
6. Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014; 43: 748-59.
7. Morley JE, Anker SD, von Haehling S. Prevalence, incidence, and clinical impact of sarcopenia: facts, numbers, and epidemiology-update 2014. J Cachexia Sarcopenia Muscle 2014; 5: 253-9.
8. Batsis JA, Mackenzie TA, Lopez-Jimenez F, Bartels SJ. Sarcopenia, sarcopenic obesity, and functional impairments in older adults: National Health and Nutrition Examination Surveys 1999-2004. Nutr Res 2015; 35:1031-9.
9. Cederholm T, Cruz-Jentoft AJ, Maggi S. Sarcopenia and fragility fractures. Eur J Phys Rehabil Med 2013; 49: 111-7.
10. Oliveira A, Vaz C. The role of sarcopenia in the risk of osteoporotic hip fracture. Clin Rheumatol 2015; 34: 1673-80.
11. Landi F, Liperoti R, Russo A, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr 2012; 31: 652-8.
12. Kim H, Suzuki T, Saito K, Kojima N, Hosoi E, Yoshida H. Long-term effects of exercise and amino acid supplementation on muscle mass, physical function and falls in community-dwelling elderly Japanese sarcopenic women: A 4-year follow-up study. Geriatr Gerontol Int 2016; 16: 175-81.
13. Baumgartner R, Koehler K, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998; 147: 755-63.
14. Laurentani F, Russo C, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol 2003; 95: 1851-60.
15. Cesari M, Kritchevsky SB, Newman AB, et al. Added value of physical performance measures in predicting adverse health related events: results from the Health, Aging and Body Composition Study. J Am Geriatr Soc 2009; 57: 251-9.
16. Lauretani F, Russo CR, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985) 2003; 95: 1851-60.
17. Volpato S, Bianchi L, Cherubini A, et al. Prevalence and clinical correlates of sarcopenia in community-dwelling older people: application of the EWGSOP definition and diagnostic algorithm. J Gerontol A Biol Sci Med Sci 2014; 69: 438-46.
18. Keller K, Engelhardt M. Strength and muscle mass loss with aging process. Age and strength loss. Muscles Ligaments Tendons J 2013: 3: 346-50.
19. Patel HP, Syddall HE, Jameson K, et al. Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: findings from the Hertfordshire Cohort Study (HCS). Age Ageing 2013; 42: 378-84.
20. Iolascon G, Giamattei MT, Moretti A, Di Pietro G, Gimigliano F, Gimigliano R. Sarcopenia in women with vertebral fragility fractures. Aging Clin Exp Res 2013; 25: S129-31.
21. Laurent MR, Dubois V, Claessens F, et al. Muscle-bone interactions: From experimental models to the clinic? A critical update. Mol Cell Endocrinol 2016; 432: 14-36. 22. Harris R, Chang Y, Beavers K, et al. Risk of fracture in women with sarcopenia, low bone mass, or both. J Am Geriatr Soc 2017; 65: 2673-8.
23. Chalhoub D, Cawthon PM, Ensrud KE, et al. Risk of non spine fractures in older adults with sarcopenia, low bone mass, or both. J Am Geriatr Soc 2015; 63: 1733-40.
24. Papageorgiou M, Sathyapalan T, Schutte R. Muscle mass measures and incident osteoporosis in a large cohort of postmenopausal women. J Cachexia Sarcopenia Muscle 2019; 10: 131-9.
25. Steihaug OM, Gjesdal CG, Bogen B, Kristoffersen MH, Lien G, Ranhoff AH. Sarcopenia in patients with hip fracture: A multicenter cross-sectional study. PLoS One 2017; 13; 12: e0184780.
26. Di Monaco M, Vallero F, Di Monaco R, Tappero R. Prevalence of sarcopenia and its association with osteoporosis in 313 older women following a hip fracture. Arch Gerontol Geriatr 2011; 52: 71-4.
27. Landi F, Liperoti R, Russo A, et al. Sarcopenia as a risk factor for falls in elderly individuals: Results from the ilSIRENTE study. Clin Nutr 2012; 31: 652-8.
28. Sornay-Rendu E, Duboeuf F, Boutroy S, Chapurlat RD. Muscle mass is associated with incident fracture in postmenopausal
women: The OFELY study. Bone 2017; 94: 108-13.
29. Lloyd BD, Williamson DA, Singh NA, et al. Recurrent and injurious falls in the year following hip fracture: a prospective study of incidence and risk factors from the sarcopenia and hip fracture study. J Gerontol A Biol Sci Med Sci 2009; 64: 599-609.
Feminists have sometimes tried to make out that Rosalind [Franklin] was an early martyr to their cause. Aaron Klug, who knew Rosalind well, once remarked to me, with reference to a book by a feminist, that “Rosalind would have hated it”. I don’t think Rosalind saw herself as a crusader or a pioneer. I think she just wanted to be treated as a serious scientist.
Las feministas han tratado a veces de pretender que Rosalind [Franklin] fue un primer mártir de su causa. Aaron Klug, que la conoció bien, una vez me señaló, refiriéndose a un libro de una feminista, que “Rosalind lo hubiera odiado”. No creo que Rosalind se viera a sí misma como una cruzada o una pionera. Pienso que ella deseaba ser tratada como una científica seria.
Francis Crick (1916-2004)
What mad pursuit. A personal view of scientific discovery. New York:Basic Books, 1988. Chapter 6. How to live with a golden helix, p 69
