LEONARDO F. L. RIZZO 1, 2, DANIELA L. MANA 1
1 Dirección Médica Química Montpellier SA, 2 División Endocrinología, Hospital de Clínicas José de San Martín, Facultad de Medicina, Universidad de Buenos Aires, Buenos Aires, Argentina
Resumen El tratamiento del hipotiroidismo tiene como objetivo restaurar el estado eutiroideo. En la mayoría de los casos los signos y síntomas del déficit tiroideo en general se resuelven, lo cual es muy gratificante para el médico tratante y en especial para los pacientes. Sin embargo, pueden coexistir situaciones especiales que potencialmente dificulten o interfieran con un tratamiento exitoso como en el caso de los pacientes ancianos, aquellos con cardiopatías, enfermedades hematológicas o dislipemia, pacientes hipotiroideos que requieran cirugía de urgencia, aquellos con insuficiencia renal crónica, o insuficiencia adrenal, entre otras. Además, se incluye el manejo del hipotiroidismo en la era del COVID-19. Algunos pacientes pueden manifestar intolerancia al tratamiento y otros, persistencia de síntomas de hipotiroidismo aun bajo un adecuado reemplazo hormonal, lo cual requerirá un abordaje especial. Estar advertido de estas situaciones especiales redundará en el beneficio del paciente y evitará fracasos o complicaciones terapéuticas.
Palabras clave: hipotiroidismo, cardiopatías, cirugía, insuficiencia renal, insuficiencia adrenal, COVID-19
Abstract The treatment of hypothyroidism is aimed at restoring the euthyroid state. In most cases, the signs and symptoms of thyroid deficiency generally resolve, which is particularly gratifying for the treating physician and mainly, for patients. However, there may be coexisting special situations that can potentially hinder or interfere with a successful treatment, as in the case of the elderly, patients suffering from heart disease, hematological diseases or dyslipidemia, hypothyroid patients who will undergo an emergency surgery, those with chronic kidney failure, or adrenal insufficiency, among others. Besides management of hypothyroidism in time of COVID-19 is also included. Some patients may experience intolerance to treatment and others persistent symptoms of hypothyroidism even under adequate replacement therapy, requiring a special approach. Being aware of these special situations will provide benefits to the patient and will also prevent treatment failure or complications.
Key words: hypothyroidism, heart disease, surgery, kidney failure, adrenal insufficiency, COVID-19
Dirección postal: Daniela Mana, Maza 578, 1220 Buenos Aires, Argentina
e-mail: daniela.mana@gmail.com
• The treatment of hypothyroidism is aimed at restoring the euthyroid state. However, there may be coexisting special situations that can potentially hinder or interfere with a successful treatment, as in the case of the elderly, patients suffering from heart disease, hematological diseases or dyslipidemia, hypothyroid patients who will undergo an emergency surgery, those with chronic kidney failure, or adrenal insufficiency, among others.
• Some patients may experience intolerance to treatment and others persistent symptoms of hypothyroidism even under adequate replacement therapy, requiring a special approach.
• Is important to know how to manage these special situations and consequently ensure the patient’s welfare and prevent failure and/or therapeutic complications.
Hypothyroidism is a condition caused by decreased production (or action) of the thyroid hormones. Its clinical expression varies from mildly elevated thyrotropin (TSH) levels in asymptomatic patients to severe hypothyroidism, which can occasionally lead to myxedema coma1.
Hypothyroidism is classified as primary when there is low thyroid gland activity; it accounts for more than 90% of the cases. Central hypothyroidism represents less than 1% of the cases2 and is caused by either low levels of TSH secretion by the anterior pituitary (secondary hypothyroidism) or low levels of thyrotropin-releasing hormone (TRH) secretion by the hypothalamus (tertiary hypothyroidism). Other far less common causes are the so-called peripheral hypothyroidism which includes the thyroid hormone resistance syndrome, defects in thyroid hormone transport and metabolism, and consumptive hypothyroidism due to peripheral consumption of thyroid hormones by tumors expressing type 3 deiodinase activity.
The causes of hypothyroidism are shown in Table 1.

Hypothyroidism is five to eight times more frequent in women than men. The most common cause of primary hypothyroidism in iodine-sufficient areas of the world is chronic autoimmune thyroiditis (Hashimoto’s disease) which has two forms: goitrous and atrophic3. But worldwide, the most common cause of hypothyroidism (and goiter) is still iodine deficiency, which is defined by an iodine intake lower than 100 mcg/day and which affects approximately two billion people.
Hypothyroidism can also be classified according to the time of onset as congenital or acquired, and based on its severity, as clinical or subclinical. Due to the broad spectrum of its clinical presentation and the non-specificity of its symptoms, the current means of diagnosing hypothyroidism is predominantly biochemical4.
Thus, clinical or overt primary hypothyroidism is defined by a high TSH concentration with low levels of free T4.
On the other hand, mild or subclinical hypothyroidism is diagnosed when the TSH level is elevated above the reference range (0.4-4.5 mU/l) and the free T4 levels remain within the normal serum range (0.7-1.7 ng/dl)5.
The treatment will be aimed at restoring the euthyroid state, which will be determined by laboratory tests and the patient’s clinical response.
The treatment of hypothyroidism is in most cases particularly rewarding for the treating physician and, mainly, for the patients since, in general, the symptoms and signs of the thyroid deficiency resolve completely.
However, special situations that can potentially hinder or interfere with a successful treatment can coexist. The aim of this review is to be aware of these special situations and therefore, provide benefits to the patient and prevent failure or therapeutic complications.
Special situations
Hypothyroidism in the elderly
Hypothyroidism is a common disorder in the elderly, affecting between 5-20% of women and between 3-8% of men6.
A key fact to take into consideration is that the normal range of reference for TSH levels used with the general population is improperly applied to test older adults, whose TSH concentrations are higher due to age-related changes. The NHANES III study showed that the upper limit of the reference range (97.5% confidence interval) increases from 3.56 mU/l in 20-29 years old to 7.9 mU/l in people more than 80 years old7.
Thus, for the diagnosis of hypothyroidism, it will be essential to consider these age-related changes in TSH levels8. 9.
On the other hand, the diagnosis of hypothyroidism in older adults can usually be delayed because their symptoms are ambiguous and atypically different from those present in young adults, and which are usually wrongly attributed to normal aging and/or to associated coexisting diseases10.
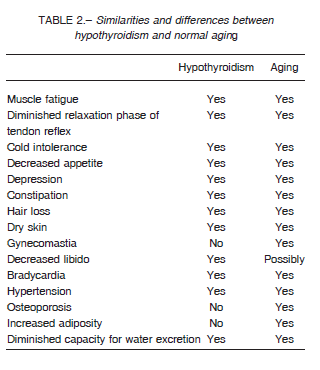
Table 2 shows the similarities and differences between hypothyroidism and normal aging.
The most common symptoms may include fatigue, weakness, dry skin, constipation, hair loss, depression, cold intolerance, loss of appetite and arthralgia11. 12.
Other common signs of the hypothyroid older patient are mainly neurological13 and cardiological14. At the neurological level, the following are included: syncope, seizures, cerebellar disorders with ataxia and intention tremor, carpal tunnel syndrome and hearing impairment of unclear pathogenesis. Some professionals attribute this to myxedema with discharge in the tympanic cavity, while others consider it secondary to edema in the Eustachian tube15. The cardiovascular manifestations are described as dyspnea, precordialgia, diastolic hypertension and heart failure10, 14.
When confronted with this multiplicity of signs and symptoms, when should we suspect hypothyroidism in the older adult?
Fundamentally, in the presence of an unexplained increase in cholesterol levels and/or creatine phosphokinase, hyponatremia, severe or previously non-existent constipation, heart failure with restrictive cardiomyopathy and macrocytic anemia as a consequence of folic acid deficiency or autoimmune gastritis co-existing with pernicious anemia10, 11.
Regarding treatment, patients over 70 years of age require a 20-30% lower dose compared to young adults (0.5-1 mcg/kg/day), due to their decreased lean body mass and reduced metabolic clearance of levothyroxine16, 17. Overtreatment and undertreatment with thyroid hormone are not uncommon, such as observed in a community study that screened 339 subjects over
65 years of age who were receiving thyroid hormone therapy. Forty one per cent of the patients showed subnormal TSH values, 16% higher TSH values, and only 43% were euthyroid18. The risk of overtreatment increases in proportion to the elapsed time since treatment initiation16. This predisposes the older adult to develop bone diseases and adverse cardiovascular effects caused by an excessive amount of thyroid hormones16. In a retrospective cross-sectional study of more than 23 000 hospitalized patients aged between 65-70, the prevalence of atrial fibrillation in patients with low TSH concentrations (< 0.4 mU/l) was 13% compared to 2.3% in euthyroid control subjects19.
Thyroid hormone replacement therapy should be initiated with minimum doses, between 12.5-25 mcg/day, and will be gradually increased at intervals of 4-6 weeks under close monitoring, given the increased sensitivity to hormone therapy of these patients and the frequent coexistence of cardiovascular and/or pulmonary diseases17. Poor compliance to treatment is commonly observed. Patients should always be asked whether they have been prescribed any other commonly used medications that interact with thyroid hormones, such as amiodarone, anticoagulants, lithium, digoxin or neuroleptics.
As regards subclinical hypothyroidism (SCH), it is currently recommended to treat those patients less than 65-70 years old who present symptoms suggestive of hypothyroidism with TSH levels above 7 mU/l. Patients with high titers of anti-thyroid peroxidase antibodies –who can progress more rapidly into clinical hypothyroidism– and those who are affected by goiter may benefit from earlier treatment.
Conversely, it is suggested that SCH should not be treated in those more than 65-70 years old with TSH levels above the upper limit of normal to 7 mU/I because TSH values in this range are age-appropriate as mentioned before. Since the upper reference range of TSH may vary between 6 to 8 mU/I in healthy octogenarians20, some authors suggest that SCH should not be treated in patients over 80 with TSH levels below 10 mUI/ml21-23.
Finally, the use of T3 and the T4 + T3 combination therapy are not advised for older adults or for patients with cardiovascular disease due to the risk of precipitating an arrhythmia or worsening the underlying cardiological condition4. 24.
Another clinical presentation, fortunately infrequent but that can be occasionally found, especially in intensive care units, is decompensated hypothyroidism or myxedema coma. This is a severe condition, with a historically high mortality rate between 40-60%, which has declined in recent years to 20-25% due to its earlier detection and given the advances of intensive care units. It is usually observed in winter, especially in elderly women, homeless and with severe, long-lasting, untreated hypothyroidism. This condition is frequently precipitated by many trigger factors such as extreme cold exposure, sedative drugs, surgery, myocardial infarction and infection. This topic has recently been extensively updated1.
Hypothyroidism and cardiovascular diseases
As hypothyroidism causes a systemic reduction of metabolism, it can be accompanied by decreased cardiac output because of reduced contractility and lower heart rate25.
The thyroid hormones exert genomic actions at heart that include the expression of specific enzymes involved in myocardial contractility and relaxation. This explains the decreased cardiac contractility observed in hypothyroidism.
Similarly, the decreased cardiac output also contributes to a reduced exercise endurance capacity.
However, signs and symptoms of heart failure are usually absent in hypothyroid patients without any other underlying heart disease. Conversely, heart failure or angina pectoris may worsen when hypothyroidism develops in patients with heart disease.
Other alterations that contribute to cardiovascular disease and that may occur with hypothyroidism are pericardial effusion, diastolic hypertension related to increased peripheral vascular resistance, hypercholesterolemia and hyperhomocysteinemia. As thyroid hormones increase oxygen consumption at the myocardial and peripheral levels, higher cardiac output is required. Thus thyroid hormone replacement can worsen the cardiovascular condition and may induce angina pectoris, myocardial infarction, arrhythmias or even sudden death26.
So levothyroxine therapy should be initiated cautiously in patients with an underlying cardiovascular disease, starting with low initial doses between 12.5 to 25 mcg daily, every three to six weeks with routine electrocardiographic and cardiac follow-up3, 4.
The ideal dose of levothyroxine for hypothyroid patients with heart disease is the one that would reverse some symptoms of hypothyroidism without worsening the underlying cardiac disease.
Therefore, the clinically satisfactory dose of thyroid hormone may not always be able to normalize the serum thyroid profile, especially TSH levels27. Patients with arrhythmias such as atrial fibrillation and Wolff-Parkinson-White syndrome are particularly sensitive to thyroid treatment and should usually be maintained with low doses of levothyroxine. These patients should be assessed more regularly, since changes in their cardiac status may lead to frequent modifications of the hormone dose. In some patients, hypothyroidism may be found simultaneously with myocardial infarction or angina pectoris. After initiating the cardiac treatment, the patient should be stabilized before starting a thyroid hormone therapy. When a coronary artery bypass graft surgery has been scheduled, it can be performed immediately; as it has been described that hypothyroidism would not affect the postoperative morbidity of these patients28.
Amiodarone-induced hypothyroidism
Amiodarone-induced hypothyroidism (AIH) usually appears within the first months of treatment (6-18 months), although it may be seen later29. It affects mainly women, older adults, subjects with pre-existing autoimmune disease and is more common in areas with sufficient iodine intake30. It is observed in about 5 to 25% of the treated patients31, and it almost always occurs in the face of a lower thyroid function such as underlying autoimmune thyroiditis, prior treatment with radioiodine, thyroid surgery or the presence of certain predisposing chronic diseases, such as thalassemia major32. The mechanism is due to the persistent antithyroid action of iodine and the antagonistic action on T3-receptors. Clinical manifestations do not differ from those observed in primary hypothyroidism.
AIH may, if prolonged or severe, induce the onset of ventricular arrhythmias such as torsades de pointes33.
Less commonly, AIH has been associated with acute kidney failure, which reverses with levothyroxine treatment and amiodarone discontinuation34.
AIH does not remit spontaneously unless the treatment with amiodarone has been withdrawn. On the other hand, in patients with previous normal thyroid function, drug discontinuation is followed by recovery of thyroid function in 60% of the cases within 2-4 months; although in the remaining 40% it can persist for 5 to 8 more months35.
Conversely, in patients with a history of thyroid dysfunction, hypothyroidism will persist even after amiodarone has been withdrawn, requiring chronic treatment with levothyroxine.
In both scenarios, if thyroid hormone treatment is executed, it is significant to be cautious with the hormone dose and to have a regular contact with the cardiologist. Anyway, it is not always necessary or advisable to treat AIH. The decision on whether to treat it will depend on the degree of hypothyroidism, the age of the patient and in particular, the underlying heart condition30, 35.
Hypothyroidism and dyslipidemia
Thyroid hormones influence the metabolism of cholesterol by controlling the activity of cholesterol ester transfer protein (CETP), liver lipase, and lipoprotein lipase (LPL).
These hormones also control the flow of bile acids and the activity and number of LDL receptors in the liver36. Therefore, a common metabolic complication of hypothyroidism is dyslipidemia37. On the other hand, a lower lipoprotein lipase activity would also be mostly responsible for the development of hypertriglyceridemia in hypothyroidism38.
Statins have become the treatment of choice for hypercholesterolemia. One of the most common risks associated with the use of statins is the development of myopathy, a risk that will be aggravated if myopathy coexists with unsuspected hypothyroidism39, 40. Remember that myopathy and cramps are common complications of untreated hypothyroidism and are also accompanied by serum elevations of creatine phosphokinase, findings that are similar to those observed with the use of statins40. For this reason, it is advisable to rule out the presence of hypothyroidism in all patients with dyslipidemia before starting a therapy with statins. The treatment of hypothyroidism normalizes dyslipidemia secondary to thyroid hormone deficiency, although to reach normal LDL cholesterol levels it would be necessary to maintain TSH concentrations less than 1.75 mU/I41. If the altered lipid profile persists 3 to 4 months after initiating the hormone replacement therapy, it will be necessary to start a treatment with hypolipemic medications.
Hypothyroidism and surgery
Although hypothyroid patients would potentially have a higher occurrence of peri and post-operative complications such as ileus, hypotension, hyponatremia, central nervous system alterations, as compared to healthy subjects42, few adverse effects have been reported in connection with hypothyroid patients undergoing surgery43. Thus, in the event of an emergency, the surgery should not be postponed, but the patient should be closely monitored to prevent complications44. The use of lower doses of anesthetics, narcotics, hypnotics and anticoagulants should be considered, in particular because the hypothyroid condition delays the metabolization of these drugs. In the case of a coronary artery bypass graft surgery or angioplasty the management should be the same28. Surgeries can be postponed until the euthyroid state is reached. In the case of patients who are receiving levothyroxine and who, for surgery reasons, cannot receive oral treatment for more than 7 to 10 days, the hormone should be administered intravenously. The dose should be approximately 70 to 80 percent of the patient’s usual oral dose because that is approximately the fraction of oral T4 that is absorbed 45.
Hypothyroidism and hematological diseases
Hypothyroidism exerts various effects on the process of hematopoiesis, blood cells and coagulation46.
Normocytic anemia is common in hypothyroidism due to decreased erythropoietin production.
Pernicious anemia is found in about 10% of the patients with autoimmune thyroiditis, whereas iron deficiency anemia arises from menstrual cycle disorders (polymenorrhea and hypermenorrhea) or malabsorption.
The correction of hypothyroidism promotes an adequate therapeutic response to iron salts47, although it should be kept in mind that patients with anemia may be particularly sensitive at the beginning of levothyroxine replacement therapy (see Thyroid hormone intolerance).
It is important to point out that levothyroxine should be taken at least four hours apart from the iron intake since iron may interfere with the absorption of the thyroid hormone.
Subclinical hypothyroidism promotes decreased fibrinolytic activity with increased risk of thrombosis, while severe hypothyroidism predisposes otherwise increased fibrinolytic activity and consequent bleeding risk48.
In turn, severe hypothyroidism can be accompanied by an acquired von Willebrand syndrome. Probably, the best explanation would be a reduced von Willebrand factor synthesis in the absence of appropriate levels of serum thyroxine49, 50. Furthermore, the therapeutic response to anticoagulants is low in hypothyroid patients.
Hormone replacement may increase the metabolism of vitamin K-dependent coagulation factors, thus increasing anticoagulant effects with the consequent risk of bleeding51.
When initiating the thyroid hormone treatment in anticoagulated patients, the anticoagulant doses should be reduced to maintain the international normalized ratio (INR) between 2 and 352. No special precautions are required when adding an anticoagulant drug to patients who are already receiving thyroid hormone therapy and are euthyroid.
Hypothyroidism and chronic kidney failure
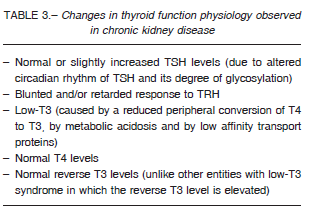
The kidneys play an important role in the metabolism, degradation and excretion of the thyroid hormones; it is therefore not surprising that kidney failure leads to alterations in thyroid physiology.
The entire hypothalamus-pituitary-thyroid axis may be affected, including alterations in hormone synthesis, distribution and excretion. It is really important to consider that the symptomatology of uremic syndrome and hypothyroidism may overlap and for this reason an accurate interpretation of the thyroid function tests will be required. Common symptoms to both entities include cold intolerance, facies, dry skin, lethargy, fatigue and constipation. The occurrence of goiter is also increased in cases of chronic renal failure53.
Patients with chronic kidney disease (CKD) have a relatively higher occurrence of thyroid pathology than the general population and of hypothyroidism in particular54, 55. This higher incidence would also be influenced by the presence of associated autoimmune entities, such as systemic lupus erythematosus and type 1 diabetes mellitus. Iodine retention induced by renal failure causes an increase in the intrathyroidal iodide pool that will be reflected in a lower radioiodine uptake.
Increased inorganic iodide may block hormone synthesis (Wolff-Chaikoff effect) which explains the aforementioned increased occurrence of goiter and hypothyroidism in patients with CKD. On the other hand, the presence of underlying thyroid autoimmunity in many cases will further increase the risk of hypothyroidism53, 55.
The treatment of hypothyroidism in patients with chronic renal failure requires a lower dose of levothyroxine.
It is essential to bear in mind that euthyroid subjects with CKD show some alterations in thyroid function tests56 which should be properly identified in order to avoid diagnostic confusion. Table 3 shows the changes in thyroid function physiology observed in CKD.
Hypothyroidism and adrenal insufficiency
Adrenal insufficiency can accompany hypothyroidism and if it is not detected, it may be exacerbated by thyroid hormone replacement, with the risk of inducing an adrenal crisis that can be fatal. Thus, in cases associated with hypothyroidism, it is necessary to start the hydrocortisone replacement first and then the levothyroxine replacement 5 to 7 days later57.
This complication can occur in three scenarios:
– Autoimmune adrenal insufficiency (Addison’s di-sease) combined with autoimmune thyroiditis, an association known as Schmidt syndrome
– Corticotropic and thyrotropic deficiencies combined in a profile of hypopituitarism
– Functional alteration of the hypothalamus-pituitaryadrenal axis as a response to stress in cases of severe hypothyroidism (as described for myxedema coma) Another situation to consider, and which can lead to confusion, is the presence of modestly increased serum levels of TSH in cases of untreated adrenal insufficiency. These levels return to normal with the correct hydrocortisone replacement treatment without the need for thyroid hormone replacement. Cortisol plays an important role in the diurnal variation of TSH levels showing lower concentrations in the morning and higher at night, which explains the mechanism by which TSH levels are elevated in cases of untreated adrenal insufficiency58. Coexisting adrenal insufficiency should be suspected in hypothyroid patients in the presence of:
a) Clinical manifestations such as fever, weight loss, nausea, vomiting, abdominal pain, hypotension, hyperpigmentation;
b) Laboratory findings such as eosinophilia, hyponatremia, hyperkalemia, hypoglycemia;
c) Other clinical findings suggesting the possibility of autoimmune polyendocrine syndromes (premature ovarian failure, type 1 diabetes, hypoparathyroidism)59 or other autoimmune entities such as vitiligo, pernicious anemia, alopecia areata;
d) History of pituitary pathology where corticotropin (ACTH) deficiency may be associated with central hypothyroidism;
e) The presence of myxedema coma
Hypothyroidism and gastroenteropathies
Digestive tract diseases can interfere with the treatment of hypothyroidism by altering, for example, the thyroid hormone requirement. The mechanism is basically caused by malabsorption and among the entities to be observed are celiac disease (to be considered when doses greater than 2 mcg/kg/day are required)60, 61, autoimmune atrophic gastritis62, 63, Helicobacter pylori infection64, gastro paresis caused by autonomic neuropathy, diarrhea caused by diabetic enteropathy, post-resection intestinal syndrome65, intestinal parasitosis66, bariatric surgery for morbid obesity67, and lactose intolerance68. Lactose intolerance or lactose malabsorption and Helicobacter pylori infection represent the most common disorders, with an overall prevalence of 68% and 48% respectively64.
Other less common digestive disorders that may be accompanied by thyroid hormone malabsorption include liver cirrhosis and ulcerative colitis. Although more evidence is necessary, it is possible that this patient population could benefit from formulations based on levothyroxine solutions to optimize their absorption64.
Thyroid hormone intolerance
Some patients report, during the first hours after taking levothyroxine, a sympathetic hyperactivity that includes anxiety, insomnia, palpitations, tremor, choking sensation, precordialgia27. These patients require very low initial doses of levothyroxine which should be increased gradually. We have observed some sporadic cases that took several months to reach normal TSH levels. In general, there is no reason to explain this phenomenon of “idiopathic sympathetic hyperactivity”.
In other cases, it may be caused by the presence of uncorrected iron-deficiency anemia that presents itself with sympathetic hyperactivity, which worsens when administering thyroid hormone47. The situation reverses after correcting the anemia and discontinuing temporarily thyroid hormone therapy, which will be restarted at low doses. Occasionally, beta blockers can be used in these cases to control the symptoms during the first few weeks of thyroid replacement treatment27.
Patients with severe hypothyroidism may also show intolerance at the beginning of the treatment. Another fact to be considered is that hypothyroidism can overlap menopause symptoms, which will become manifest or exacerbated with thyroid hormone replacement.
As mentioned above, older adults and patients with coronary heart disease and arrhythmias are also more sensitive to thyroid hormone treatment.
Persistent symptoms of hypothyroidism even under adequate replacement therapy
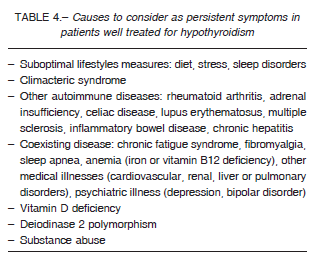
Even though the majority of patients rendered euthyroid with L-T4, some still experience residual symptoms of hypothyroidism, have metabolic abnormalities, or are otherwise dissatisfied with their treatment despite normalized TSH levels69-72. Decreased quality of life in biochemically euthyroid patients with hypothyroidism could have several potential causes such as physical, psychological, social, economic, disease related factors, with treatment for their disease perhaps not being the main relevant factor. For example, with respect to physical factors, impaired quali-ty of life has been found to be associated with increased body mass index in patients treated for hypothyroidism71. In a recent electronic survey carried out by the American Thyroid Association of over 11000 hypothyroid patients, 95% women, the mean treatment satisfaction score was 5 on a visual analog scale of 1-1070. Interestingly, satisfaction scores were significantly higher in patients who were taking L-T4/L-T3 combination therapy or desiccated thyroid. The most common persistent symptoms in this survey were fatigue/low energy, body weight concerns, and memory and mood problems70. Once it has been verified that the dose of levothyroxine and patient’s compliance with the treatment are adequate, a few patients may feel better by minimal increases of thyroid hormone to reach TSH levels in the low normal range.
For whom still feel symptoms of hypothyroidism even with this last approach, the explanations that have been proposed are the inadequacy of T4 to physiologically res-tore tissue thyroid hormone levels to normal (see below) or factors related to the autoimmune thyroid disease itself such as inflammation in other tissues from autoimmune thyroid antibodies. Many patients with hypothyroidism may also have other chronic conditions, and there may be overlap in the symptoms associated with these conditions. For example, rheumatologic disorders may be associated with similar symptoms such as fatigue, waking up tired and fuzzy thinking or forgetfulness. Some studies also suggest that individuals with hypothyroidism are more likely to be diagnosed with disorders such as anxiety and depression.
Thus, it is very important for the patient to understand and be well informed that symptoms frequently experienced by hypothyroid patients are nonspecific and could be due to other conditions unrelated to thyroid function3. So before considering alternate treatment strategies it is necessary to rule out other potential causes for persistent symptoms such as suboptimal lifestyle habits (e.g., diet, stress, sleep disorders), coexisting disease (e.g., chronic fatigue syndrome, depression, sleep apnea, autoimmune diseases), among others (Table 4). When other potential causes for persistent symptoms have been ruled out, combination therapy with L-T4/L-T3 is an approach that is gaining support among physicians73. Special populations of hypothyroid patients may be favored with this therapy strategy such as athyreotic and radioablated patients and those with polymorphisms in iodothyronine deiodinase 2, DIO2 gene. Lower serum T3 levels are often seen in these cases and may be due to inadequate peripheral deiodination of T4 to T374. If combination therapy is adopted, careful attention to maintaining serum TSH within the reference range is critical to avoid the known toxicities of thyroid hormone overtreatment.
Most guidelines that support combination therapy in patients with persistent symptoms of hypothyroidism discourage this approach in pregnant women, older adults and pediatric population due to the effects of rapid serum T3 fluctuations and potential adverse events72.
Hypothyroidism in the era of COVID-19
The World Health Organization (WHO) declared infection by the SARS-CoV-2 causing COVID-19 disease a global pandemic on March 2020.
Hypothyroidism is usually a chronic condition whose main etiology is autoimmune thyroiditis. Until now there is no evidence that patients with existing autoimmune thyroid disease are more susceptible to contracting viral illnesses including SARS- CoV-2 or that they are at risk of developing more severe COVID-19 disease75.
SARS-CoV-1 has been known to affect the hypothalamic-pituitary-adrenal axis (HPA) causing transient hypocortisolism. Less commonly, the hypothalamic-pituitary-thyroid axis is affected leading to central hypothyroidism. Both reversible hypophysitis and a direct hypothalamic effect have been reported as possible mechanisms76.
A report on thyroid function tests in 48 patients infected with SARS-CoV-1 showed reduced free T3 and free T4 in 94% and 46% of patients, respectively. Serum TSH levels were also reduced in these patients, raising the possibility of either central hypothyroidism or the “sick euthyroid” syndrome. In another follow-up study that evaluated endocrine disorders among 61 patients who had SARS, 2 patients were identified as having subclinical thyrotoxicosis, 3 had central hypothyroidism, and 1 had primary hypothyroidism with positive thyroid autoantibodies. Therefore, previously undiagnosed primary hypothyroidism and recovering central hypothyroidism or sick euthyroidism are possibilities that should be considered.
Acute and chronic phases of critical illness have different effects on the thyroid axis. Sick euthyroid syndrome or “nonthyroidal illness syndrome” can manifest in patients with COVID-19, especially during the acute and recovery phase of the illness, which will further complicate their management. This mechanism is complex. During an acute illness, changes in thyroid hormone binding, cellular uptake, and decreased activity of type 1 deiodinase enzyme leading to decreased T4 to T3 conversion, can occur. Type 1 deiodinase enzyme activity is reduced as it is influenced by various substances including circulating cortisol, cytokines, endogenous free fatty acids, and various drugs used in management. Increased T3 catabolism in peripheral tissues can occur because of increased activity of type 3 deiodinase. The cumulative effect is low circulating T3 levels. In addition to these changes, down-regulation of the hypothalamic-pituitary axis leads to low circulating TSH and T4 levels during the course of illness. Differentiating this from central hypothyroidism might be difficult in the acute stage and may require reevaluation later. If nonthyroidal illness syndrome is suspected, therapy with thyroid hormone is not currently recommended because of lack of clinical benefit and safety concerns76, 77.
A recent report from Italy on a young female who recovered from SARS-CoV-2 describes the first reported case of subacute thyroiditis chronologically related to this viral infection. With the known association of subacute thyroiditis with preceding viral infections, it is possible that the thyroiditis is etiologically related to SARS-CoV-2 infection78.
With respect to management of hypothyroidism there are no particular recommendations or changes relating to the diagnosis and treatment of this entity during COVID-19 pandemic. Patients may be instructed to continue the same form and dosage of thyroid hormone replacement therapy79.
In case of infection with SARS-CoV2 in a patient with hypothyroidism who develops critical respiratory illness, and especially when assisted mechanical ventilation is required, it will not be possible to take oral levothyroxine.
In these situations, injectable levothyroxine should be administered, intravenously, at a dose equivalent to 70 to 80% of that which the patient received orally.
Conclusions
Treatment of hypothyroidism is in most cases easy, successful and rewarding for the medical doctor and the patients, since signs and symptoms of thyroid hormone deficiency reverse completely in the vast majority of the cases.
However, special situations can coexist and may hinder or interfere with the treatment of hypothyroidism.
Therefore, it is important to know how to manage these special situations and consequently ensure the patient’s welfare and prevent failure and/or therapeutic complications.
Conflict of interest: Leonardo F. L. Rizzo is the Medical Director of Química Montpellier S.A. and Daniela Mana is Medical Consultants for Química Montpellier S.A.
References
1. Rizzo LFL, Mana DL, Bruno OD, Wartofsky L. Coma mixedematoso. Medicina (B Aires) 2017; 77: 321-8.
2. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet 2017; 390: 1550-62.
3. Biondi B, Cooper DS. Thyroid hormone therapy for hypothyroidism. Endocrine 2019; 66:18-26.
4. Biondi B, Wartofsky L. Treatment with thyroid hormone. Endocr Rev 2014; 35: 433-512.
5. Garber JR; Cobin RH, Gharib H, et al. Clinical Practice Guidelines for hypothyroidism in adults cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Thyroid 2012; 22: 1200-35.
6. Laurberg P, Andersen S, Bülow Pedersen I, Carle A. Hypothyroidism in the elderly: pathophysiology, diagnosis, and treatment. Drugs Aging 2005; 22: 23-8.
7. Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002; 87: 89-99.
8. Bremner AP, Feddema P, Leedman PJ, et al. Agerelated changes in thyroid function: a longitudinal study of a community-based cohort. J Clin Endocrinol Metab 2012; 97: 1554-62.
9. Vadiveloo T, Donnan PT, Murphy MJ, Leese GP. Ageand gender-specific TSH reference intervals in people with no obvious thyroid disease in Tayside, Scotland: the Thyroid Epidemiology, Audit, and Research Study (TEARS). J Clin Endocrinol Metab 2013; 98: 1147-53.
10. Duntas LH, Yen PM. Diagnosis and treatment of hypothyroidism in the elderly. Endocrine 2019; 66: 63-9.
11. Chiovato L, Mariotti S, Pinchera A. Thyroid diseases in the elderly. Bailliere’s Clin Endocrinol Metab 1997; 11: 251-70.
12. Mariotti S, Franceschi C, Cossarizza A, Pinchera A. The Aging thyroid. Endocr Rev 1995;16: 686-715.
13. Boyages SC. The neuromuscular system and brain in hypothyroidism. In: Braverman LE, Utiger RD, eds. Werner and Ingbar’s The Thyroid: A Fundamental and Clinical Text, 8th ed., Lippincott, Williams & Wilkins, 2000, p 803-10.
14. Ladenson PW. Recognition and management of cardiovascular disease related to thyroid dysfunction. Am J Med 1990; 88: 638-41.
15. Bruno OD. Neurological manifestations of endocrinemetabolic disorders. In: Micheli FE, Fernández Pardal MM, eds. Neurología del anciano. Ed. Panamericana, 1996, p 379-88.
16. Iglesias P, Diez JJ. Thyroid dysfunction and kidney disease. Eur J Endocrinol 2009; 160: 503-15.
17. Ruggeri RM, Trimarchi F, Biondi B. L-thyroxine replacement therapy in the frail elderly: a challenge in clinical practice. Eur J Endocrinol 2017; 177: R199-217.
18. Somwaru LL, Arnold AM, Joshi N, Fried LP, Cappola AR. High frequency of and factors associated with thyroid hormone over-replacement and underreplacement in men and women aged 65 and over. J Clin Endocrinol Metab 2009; 94: 1342-5.
19. Auer J, Scheibner P, Mische T, Langsteger W, Eber O, Eber B. Subclinical hyperthyroidism as a risk factor for atrial fibrillation. Am Heart J 2001; 142: 838-42.
20. Cappola AR. The thyrotropin reference range should be changed in older patients. JAMA 2019 Oct 30. doi: 10.1001/jama.2019.14728. [Epub ahead of print]
21. Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frölich M, Westendorp RG. Thyroid status, disability and cognitive function, and survival in old age. JAMA 2004; 292: 2591-99.
22. Cooper DS. Thyroid disease in the oldest old: the exception to the rule. JAMA 2004; 292: 2651-54.
23. Mooijaart SP, Du Puy RS, Stott DJ, et al Association between levothyroxine treatment and thyroid-related symptoms among adults aged 80 years and older with subclinical hypothyroidism. JAMA 2019 Oct 30:1-11.doi: 10.1001/jama.2019.17274.[Epub ahead of print]
24. Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American Thyroid Association task force on thyroid hormone replacement. Thyroid 2014; 24: 1670-751.
25. Danzi S, Klein I. Thyroid disease and the cardiovascular system. Endocrinol Metab Clin N Am 2014; 43: 517-28.
26. Fazio S, Palmieri EA, Lombardi G, Biondi B. Effects of thyroid hormone on the cardiovascular system. Recent Prog Horm Res 2004; 59: 31-50.
27. Roberts CG, Ladenson PW. Hypothyroidism. Lancet 2004; 363: 793-803.
28. Becker C. Hypothyroidism and atherosclerotic heart disease: pathogenesis, medical management, and the role of coronary artery bypass surgery. Endocr Rev 1985; 6: 432-40.
29. Trip MD, Wiersinga WM, Plomp TA. Incidence, predictability and pathogenesis of amiodarone-induced thyrotoxicosis and hypothyroidism. Am J Med 1991; 91: 507-11.
30. Rizzo LF, Bruno OD. Amiodarone and thyroid dysfunction. Medicina (B Aires) 2012; 72: 63-74.
31. Batcher EL, Tang XC, Singh BN, et al. Thyroid function abnormalities during amiodarona therapy for persistent atrial fibrillation. Am J Med 2007; 120: 880-5.
32. Mariotti S, Loviselli A, Murenu S, et al. High prevalence of thyroid dysfunction in adult patients with betathalassemia major submitted to amiodarone treatment. J Endocrinol Invest 1999; 22: 55-63.
33. Conen-Lehman J, Dahl P, Danzi S, Klein I. Effects of amiodarone therapy on thyroid function. Nat Rev Endocrinol 2010; 6: 34-41.
34. Luciani R, Falcone C, Principe F, Punzo G, Mené P. Acute renal failure due to amiodarone- induced hypothyroidism. Clin Nephrol 2009; 72: 79-80.
35. Basaria S, Cooper DS. Amiodarone and the thyroid. Am J Med 2005; 118: 706-14.
36. Biondi B. Persistent dyslipidemia in patients with hypothyroidism: a good marker for personalized replacement therapy? J Clin Endocrinol Metab 2019; 104: 624-7.
37. Moon JH, Kim HJ, Kim HM, et al. Decreased expression of hepatic low-density lipoprotein receptor-related protein 1 in hypothyroidism: a novel mechanism of atherogenic dyslipidemia in hypothyroidism. Thyroid 2013; 23: 1057-65.
38. Gjedde S, Gormsen LC, Rungby J, et al. Decreased lipid intermediate levels and lipid oxidation rates despite normal lipolysis in patients with hypothyroidism. Thyroid 2010; 20: 843-9.
39. Venero CV, Thompson PD. Managing statin myopathy. Endocrinol Metab Clin North Am 2009; 38: 121-36.
40. Robison CD, Bair TL, Horne BD, et al. Hypothyroidism as a risk factor for statin intolerance. J Clin Lipidol 2014; 8: 01-7.
41. Peterson SJ, McAninch EA, Bianco AC. Is a normal TSH synonymous with “euthyroidism” in levothyroxine monotherapy? J Clin Endocrinol Metab 2016; 101: 4964-73.
42. Stathatos N, Wartofsky L. Perioperative management of patients with hypothyroidism. Endocrinol Metab Clin North Am 2003; 32: 503-18.
43. Weinberg AD, Brennan MD, Gorman CA, Marsh HM, O’Fallon WM. Outcome of anesthesia and surgery in hypothyroid patients. Arch Intern Med 1983; 143: 893-7.
44. Ladenson PW, Levin AA, Ridgway EC, Daniels GH. Complications of surgery in hypothyroid patients. Am J Med 1984; 77: 261-6.
45. Hays MT, Nielsen KR. Human thyroid absorption: age effects and methodological analyses. Thyroid 1994; 4: 55-64.
46. Marqusee E, Mandel SJ. The blood in hypothyroidism. In: Braverman LE, Utiger RD (eds) Werner and Ingbar´s The Thyroid. A Fundamental and Clinical Text 9th ed. Lippincott Williams & Wilkins, Philadelphia, 2005, p 803-5.
47. Cinemre H, Bilir A, Gokosmanoglu F, Bahcebasi T. Hematologic effects of levothyroxine in iron-deficient subclinical hypothyroid patients: A randomized, doubleblind, controlled study. J Clin Endocrinol Metab 2009; 94: 151-6.
48. Franchini M, Montagnana M, Manzato F, Vescovi PP. Thyroid dysfunction and hemostasis: An issue still unresolved. Semin Thromb Hemost 2009; 35: 288-94.
49. Marongiu F, Cauli C, Mariotti S. Thyroid, hemostasis and thrombosis. J Endocrinol Invest 2004; 27: 1065-71.
50. Manfredi E, van Zaane B, Gerdes VE, Brandjes DP, Squizzato A. Hypothyroidism and acquired von Willebrand’s syndrome: a systematic review: Haemophilia 2008; 14: 423-33.
51. Gullu S, Sav H, Kamel N. Effects of levothyroxine treatment on biochemical and hemostasis parameters in patients with hypothyroidism. Eur J Endocrinol 2005; 152: 355-61.
52. Bucerius J, Joe AY, Palmedo H, Reinhardt MJ, Biersack HJ. Impact of short-term hypothyroidism on systemic anticoagulation in patients with thyroid cancer and coumarin therapy. Thyroid 2006; 16: 369-74.
53. Kaptein EM. Thyroid hormone metabolism and thyroid diseases in chronic renal failure. Endocr Rev 1996; 17: 45-63.
54. Lo JC, Chertow GM, Go AS, Hsu CY. Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney Int 2005; 67: 1047-52.
55. Iglesias P, Diez JJ. Thyroid dysfunction and kidney disease. Eur J Endocrinol 2009; 160: 503-15.
56. Lim VS. Thyroid function in patients with chronic renal failure. Am J Kidney 2001; 38: S80-4.
57. Arima H, Iwama S, Inaba H, et al. Management of immune-related adverse events in endocrine organs induced by immune checkpoint inhibitors: clinical guidelines of the Japan Endocrine Society. Endocr J 2019; 66: 581-6.
58. Samuels MH. Effects of variations in physiological cortisol levels on thyrotropin secretion in subjects with adrenal insufficiency: a clinical research center study. J Clin Endocrinol Metab 2000; 85: 1388-93.
59. Kahaly GJ, Frommer L. Polyglandular autoimmune syndromes. J Endocrinol Invest 2018; 41: 91-8.
60. Elfström P, Montgomery SM, Kämpe O, Ekbom A, Ludvigsson JF. Risk of thyroid disease in individuals with celiac disease. J Clin Endocrinol Metab 2008; 93: 3915-21.
61. Jiskra J, Límanová Z, Vaníčkova Z, Kocna P. Ig and IgG antigliadin, Ig anti-tissue transglutaminase and antiendomysial antibodies in patients with autoimmune thyroid diseases and their relationship to thyroidal replacement therapy. Physiol Res 2003; 52: 79- 88.
62. Lahner E, Centanni M, Agnello G, et al. Occurrence and risk factors for autoimmune thyroid disease in patients with atrophic body gastritis. Am J Med 2008; 121: 136-41.
63. Checchi S, Montanaro A, Pasqui L, et al. L-thyroxine requirement in patients with autoimmune hypothyroidism and parietal cell antibodies. J Clin Endocrinol Metab 2008; 93: 465-69.
64. Castellana M, Castellana C, Giovanella L, Trimboli P. Prevalence of gastrointestinal disorders having an impact on tablet levothyroxine absorption: should this formulation still be considered as the first line therapy? Endocrine 2020; 67: 281-90.
65. Bevan JS, Munro JF. Thyroxine malabsorption following intestinal bypass surgery. Int J Obes 1986; 10: 245-6.
66. Seppel T, Rose F, Schlaghecke R. Chronic intestinal giardiasis with isolated levothyroxine malabsorption as reason for severe hypothyroidism-implications for localization of thyroid hormone absorption in the gut. Exp Clin Endocrinol Diabetes 1996; 104:180-2.
67. Padwal R, Brocks D, Sharma AM. A systematic review of drug absorption following bariatric surgery and its theoretical implications. Obes 2010; 11: 41-50.
68. Muñoz-Torres M, Varsavsky M, Alonso G. Lactose intolerance revealed by severe resistance to treatment with levothyroxine. Thyroid 2006; 16: 1171-3.
69. McAninch EA, Rajan KB, Miller CH, Bianco AC. Systemic thyroid hormone status during levothyroxine therapy in hypothyroidism: a systematic review and meta-analysis. J Clin Endocrinol Metab 2018; 103: 4533-42.
70. Peterson SJ, Cappola AR, Castro MR, et al. An online survey of hypothyroid patients demonstrates prominent dissatisfaction. Thyroid 2018; 28: 707-21.
71. Jonklaas J. Persistent hypothyroid symptoms in a patient with a normal thyroid stimulating hormone level. Curr Opin Endocrinol Diabetes Obes 2017; 24: 356-63.
72. Ettleson MD, Bianco AC. Individualized therapy for hypothyroidism: Is T4 enough for everyone. J Clin Endocrinol Metab 2020; 105: 1-15.
73. Jonklaas J, Tefera E, Shara N. Physician choice of hypothyroidism therapy: influence of patient characteristics. Thyroid 2018; 28: 1416-24.
74. Bianco AC. Pathophysiological relevance of deiodinase polymorphism. Curr Opin Endocrinol Diabetes Obes 2018; 25: 341-6.
75. Boelaert K, Visser WE, Taylor TN, et al. Endocrinology in the time of COVID-19. Management of hyperthyroidism and hypothyroidism. Eur J Endocrinol 2020; 183: G33-9.
76. Somasundaram NP, Ranathunga I, Ratnasamy V, et al. The Impact of SARS-Cov-2 Virus Infection on the Endocrine System. J Endocr Soc 2020; 4:1-22.
77. Caron P. Thyroid Disorders and Sars-Cov-2 Infection: from pathophysiological mechanism to patient management. Ann Endocrinol (Paris) 2020; S0003-4266(20)31214-2. doi: 10.1016/j.ando.2020.09.001.
78. Dworakowska D, Grossman AB. Thyroid disease in the time of COVID-19. Endocrine 2020; 68: 471-4.
79. American Thyroid Association (ATA). Novel Coronavirus (COVID-19) and the Thyroid: Frequently Asked Questions. May 2020. En:https://www.thyroid.org/covid-19/coronavirus-frequently-asked-questions/; accessed August 2020.
