SANTIAGO E. MELENDI, MARÍA M. PÉREZ, CINTIA E. SALAS, MARIANA F. HAEDO, FRANCO B. XAVIER, JANDRY D. SALTOS NAVARRETE, CAMILA AGUIRRE, MARÍA L. BALETA, FACUNDO J. BALSANO, MARIANO G. CALDANO, MARÍA G. COLIGNON, THAYANA DE OLIVEIRA BRASIL, NICOLÁS DE WOLODIMEROFF, ANDREA I. DÉRAMO AQUINO, ANA G. FERNÁNDEZ DE CÓRDOVA, MARÍA B. FONTAN, FLORENCIA I. GALVAGNO, NOELIA S. ITURRIETA ARAYA, VOLGA S. MOLLINEDO CRUZ, AGUSTÍN OLIVERO, IGNACIO PESTALARDO, MARÍA RICCIARDI, MARÍA L. VERA RUEDA, MARÍA C. VILLAVERDE, MARCELA LAUKO MAURI, CARLOS UJEDA, ROCÍO LEIS
Hospital General de Agudos Carlos G. Durand, Buenos Aires, Argentina
Resumen Las características clínicas del COVID-19 difieren sustancialmente según la presencia (o ausencia) de neumonía viral. El objetivo de este artículo fue describir las características clínicas de los pacientes con COVID-19 internados en el servicio de Clínica Médica, divididos en pacientes con y sin neumonía. Fue un estudio de cohorte prospectivo, con base en un único centro, realizado en un hospital público de la ciudad de Buenos Aires: Hospital General de Agudos Carlos G. Durand. La recolección basal de datos se realizó dentro de las 48 horas del ingreso y los pacientes fueron seguidos hasta el alta o la muerte hospitalaria. Las características epidemiológicas, clínicas, de laboratorio y radiológicas junto con los datos del tratamiento se obtuvieron de la historia clínica. De los 417 incluidos, 243 (58.3%) tenían neumonía. La mediana de edad fue de 43 años (RIC: 32-57) y 222 (53.2%) eran mujeres. La tasa global de letalidad fue del 3.8%. Ninguno de los pacientes con COVID-19 sin neumonía desarrolló enfermedad crítica, requirió ventilación mecánica invasiva ni falleció durante la hospitalización. Sin embargo, 7 (4%) desarrollaron enfermedad grave durante el seguimiento. Entre aquellos con neumonía COVID-19, la tasa de mortalidad hospitalaria fue del 6.6%, se desarrolló enfermedad grave en 81 (33.3%), enfermedad crítica en 23 (9.5%) y 22 (9.1%) fueron trasladados a la unidad de cuidados intensivos. Los pacientes con COVID-19 sin neumonía presentaron buen pronóstico; sin embargo, incluso en este grupo, se observaron algunos con progresión clínica desfavorable, por lo que se requirió seguimiento adecuado. En los pacientes con neumonía por COVID-19, el desarrollo de enfermedad crítica fue frecuente y las tasas observadas en esta cohorte proporcionan una caracterización sólida de las características clínicas de los pacientes con COVID-19 en una importante ciudad de América del Sur.
Palabras clave: infecciones por coronavirus, neumonía, pacientes internados, medicina interna, epidemiología
Abstract The clinical features of COVID-19 differ substantially upon the presence (or absence) of viral pneumonia. The aim of this article was to describe the clinical characteristics of COVID-19 patients admitted to the Internal Medicine ward, as divided into those with and without pneumonia. This single-center prospective cohort study was conducted in a tertiary teaching public hospital in Buenos Aires City named Hospital General de Agudos Carlos G. Durand. Baseline data collection was performed within 48 hours of admission and patients were followed until discharge or in-hospital death. Epidemiological, clinical, laboratory, and radiological characteristics together with treatment data were obtained from the medical records. Of the 417 included, 243 (58.3%) had pneumonia. Median age was 43 years (IQR:32-57) and 222 (53.2%) were female. The overall crude case-fatality rate was 3.8%. None of the COVID-19 patients without pneumonia developed critical disease, required invasive mechanical ventilation nor died during hospitalization. However, 7 (4%) developed severe disease during follow-up. Among patients with COVID-19 pneumonia, in-hospital mortality rate was 6.6%, severe disease developed in 81 (33.3%), critical disease in 23 (9.5%), and 22 (9.1%) were admitted to the intensive care unit. A largely good prognosis was observed among COVID-19 patients without pneumonia, still, even among this group, unfavorable clinical progression can develop and should be properly monitored. Critical illness among patients with COVID-19 pneumonia was frequent and observed rates from this cohort provide a sound characterization of COVID-19 clinical features in a major city from South America.
Key words: coronavirus infections, pneumonia, inpatients, internal medicine, epidemiology
Postal address: Santiago E. Melendi, Av. Díaz Vélez 5044, Piso 3, 1405 Buenos Aires, Argentina
e-mail: santiagomelendi@gmail.com
Current knowledge
• The clinical features of COVID-19 differ substantially upon the presence (or absence) of viral pneumonia.
Article contribution to current knowledge
• COVID-19 crude case-fatality rate in the internal medicine ward was 3.8%.
• None of the COVID-19 patients without pneumonia required ICU nor died. However, 4% developed severe disease.
• Critical illness developed in 9.5% of patients with COVID-19 pneumonia
• A higher prevalence of comorbidities was observed among patients with pneumonia.
The first report of COVID-19 cases in Argentina was on March 3, 2020, two months after the first virus identification in Wuhan, China 1, 2. With almost 3 million inhabitants, the City of Buenos Aires constituted the outbreak epicenter during its initial stages and, up to November 26, 2020 roughly 157 000 cases of COVID-19 have been confirmed.
Patients with a mild clinical presentation (absence of viral pneumonia and hypoxia) usually do not require hospitalization, and should therefore be able to undergo their illness at home or temporary treatment centers (field-hospitals)3, 4. However, several COVID-19 patients with mild disease did not meet eligibility criteria for home isolation or management in field-hospitals (i.e.: presence of risk-conferring comorbidities or family members with admission criteria and no possibility of separate isolation of the other members).
Under this scenario, we faced a novel model of care in the internal medicine ward (IMW) of a General Hospital, characterized by the coexistence of COVID-19 inpatients with well-defined hospitalization criteria, together with clinically stable confirmed cases that would have been treated in an outpatient setting.
Moreover, although clinical characteristics of COVID-19 hospitalized patients have been described in many studies from the northern hemisphere5, 6, reports from South American countries are scarce, despite the extensive burden of disease faced by the countries in the region.
This study describes the clinical characteristics of COVID-19 adult inpatients, as divided into those with pneumonia and those without. It is based on patients admitted to the IMW of one of the tertiary referral public hospitals in the City of Buenos Aires during the first months of the epidemic.
Materials and methods
This single-center observational prospective cohort study was conducted in the IMW of a tertiary teaching public hospital in Buenos Aires City named Hospital General de Agudos Carlos G. Durand. It was designated as a referral hospital for COVID-19 patients within the city’s public health system and has in-house availability of real time PCR (RT-PCR) for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The 50-bed IMW was allocated to the treatment of non-ventilated COVID-19 patients. Hospitalized patients could have COVID-19 pneumonia or else mild disease without criteria for outpatient management. Patients without pneumonia were admitted to the ward because of one of the following reasons: a) risk conferring comorbidities; b) absence of proper conditions for home care; c) isolation until viral testing for SARS-CoV-2 results were available, including asymptomatic individuals with recent known or suspected exposure to SARS-CoV-2. After confirming SARS-CoV-2 infection, hospitalized patients without pneumonia, hypoxemia and no co-morbidities were transferred to extra-hospital facilities or home care3.
This study prospectively included all patients (aged ≥ 18 years) admitted to the IMW between April 7, 2020 (i.e.: the date in which the first patient was admitted) and July 31, 2020, with laboratory-confirmed COVID-19 by RT-PCR of nasopharyngeal and oropharyngeal swab samples. Pneumonia diagnosis was defined as radiologically confirmed if clinical and radiological evidence (chest x-ray) was present within 48 hours from admission. Clinically-defined pneumonia was established if determined by the treating physician based on physical examination in absence of infiltrates on the chest x-ray7. Patients without pneumonia were defined as those without compatible clinical signs and absence of infiltrates on the admission chest x-ray. We excluded those that developed COVID-19 infection during hospitalization longer than 14 days for another pre-existing reason, inpatient stays shorter than 24 hours, and patients transferred from the intensive care unit (ICU) without prior admission into the IMW (since selection bias would have been introduced by not accounting for nonsurviving ICU patients). Follow-up time was right-censored on August 30, 2020 so that all patients have at least 30 days of follow-up. No sample size calculation was performed, and no upper limit of patients to include was established for this cohort.
Epidemiological, clinical, laboratory, and radiological characteristics together with treatment data were obtained from the medical records, using electronic data collection forms designed for this study, which were adapted from the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC-WHO) case report forms for clinical characterization of acute respiratory infection8.
Baseline data collection was performed within 48 h of admission and patients were followed until discharge or inhospital death. All data came from usual care and the study did not have any influence on the diagnostic studies performed or treatment plan. Data collection was performed by a team of medical doctors, previously trained and certified in the standardized operational procedures of the study. All forms were audited by a second reviewer and, in case of discrepancies, a third reviewer was called for the final adjudication.
Prospectively included patients that were transferred to the ICU remained monitored for outcome assessment until dead or discharge. Those who were transferred to another institution were censored on the last follow-up date.
The primary endpoint was in-hospital death. Secondary outcomes include admission to ICU, requirement of invasive mechanical ventilation (IMV) and the presence of severe and critical illness. The severity of the disease was determined on admission and during the most severe phase during hospitalization.
Severe disease was defined by the presence of at least one of the following conditions: (a) Respiratory rate ≥ 30/min; (b) Arterial partial pressure of oxygen (PaO2) < 70 mmHg or peripheral oxygen saturation (SpO2) ≤ 93% on room air or PaO2 FiO2 ≤ 300; (c) Lung involvement > 50% on chest radiography9. Critical disease was defined by satisfying one of the following conditions: (a) Respiratory failure; (b) Mechanical ventilation; (c) Shock.
Baseline data collection included sociodemographic characteristics, known medical history and co-morbidities, illness onset and symptoms, vital signs (on admission to the IMW), and biochemical studies performed within 48h of admission to the hospital. Health insurance included the presence of social security or private health coverage. Health worker was a self-reported variable defined as any activity that includes care/contact with patients or carried out in clinical or microbiology laboratories. Exposure history was defined as known exposure in the last 14 days to COVID-19 cases or attendance to healthcare centers providing care for COVID-19 patients.
The following predictive scores were calculated using baseline data: Charlson comorbidity index 10, CURB-65 11, Sequential Organ Failure Assessment (SOFA) Score12, and Glasgow coma scale13.
During follow-up, data regarding supportive care and pharmacological treatment received during hospitalization was collected.
Sepsis was defined as a variation of 2 or more points on the SOFA score or record of this diagnosis in medical charts. Septic shock and acute respiratory distress syndrome were determined based on the presence of this diagnosis in medical records. Coagulopathy was defined as a 5-second extension of the activated partial thromboplastin time (aPTT) or prothrombin time (PT) <50%14. Acute kidney injury (AKI) was defined as an increase in serum creatinine level ≥ 0.3 mg/dl or ≥ 1.5 times from baseline15.
Sociodemographic, clinical, imaging, laboratory, and outcome results were compared between patients with or without pneumonia. Categorical data was presented as counts and percentages while continuous data was expressed as median with interquartile range 0.25-0.75 (IQR) or mean with standard deviation (SD). We used the Mann-Whitney U test, t test or χ² test to compare differences between both groups where appropriate. As not all patients underwent laboratory testing, sample size for each determination was reported. No imputation strategy for missing data was used. A two-sided α of less than 0.05 was considered statistically significant.
Statistical analyses were performed in September 2020 using the STATA software version 14.2 (College Station, TX, USA).
This study was approved by the institutional review board at Hospital General de Agudos Carlos G. Durand. The requirement for informed consent was waived because only routinely collected data from usual care was used. The study protocol has been already published16. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline was used for this article17.
Results
Between April 7 and July 31, 2020, 675 patients were admitted to the IMW, of which 417 (61.8%) had laboratoryconfirmed COVID-19 and met the inclusion criteria for this study. Of them, 243 (58.3%) had pneumonia diagnosis within 48hs of admission. Most pneumonia diagnoses were radiologically-confirmed (226/243; 93.0%) while the remaining cases were clinically-defined.
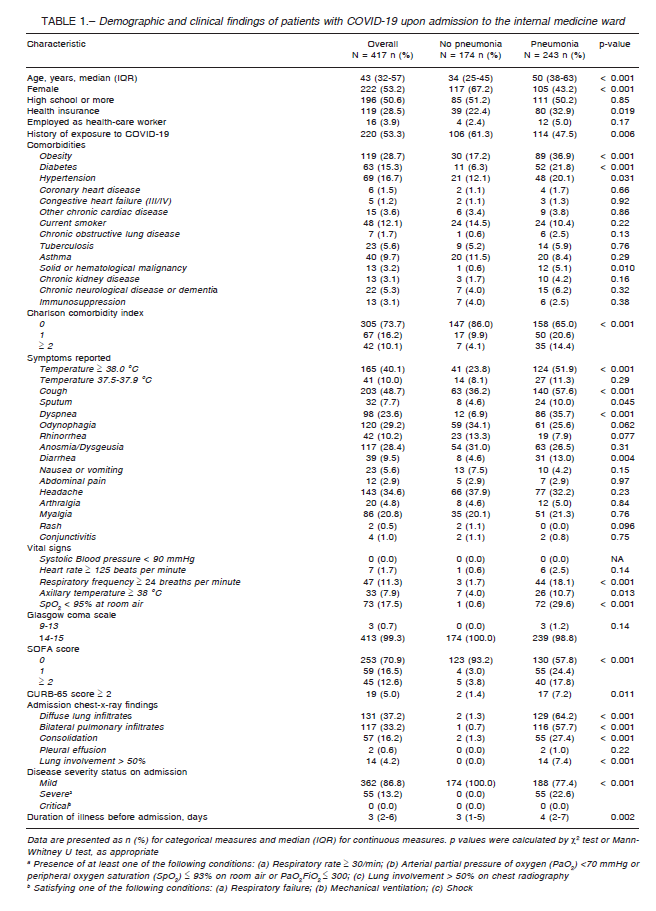
Demographic and clinical findings of patients are depicted in Table 1. The median age of patients was 43 years (IQR: 32-57), ranging from 18 to 97 years. Most patients were younger than 65 years (352/417; 84,4%) and female (222/417; 53.2%). Among patients with pneumonia, older age and a higher proportion of males was observed (Table 1).
Overall, 243 (63.8%) had at least one comorbidity with obesity being the most frequent one, in nearly one-third of patients, followed by hypertension. Most comorbidities prevailed in those with pneumonia, with an accentuated difference for obesity, diabetes, hypertension, and a history of malignant tumors (Table 1).
A higher proportion of febrile (≥ 38.0 °C) patients was observed among those with pneumonia, together with sputum production, dyspnea, and diarrhea (Table 1). There were 31 (7.4%) asymptomatic. While most of them had no evidence of pneumonia, 8 (3.3%) patients with pneumonia were asymptomatic, detected in epidemiological investigations from nursing homes (n = 6), or as part of epidemiological surveillance in vulnerable neighborhoods (n = 2). All patients without pneumonia had mild disease upon admission while 55 (22.6%) of pneumonia patients were admitted with severe disease (Table 1). None of the included had critical disease upon admission since those cases were directly admitted to the ICU upon arrival to the emergency department or within 24 h from admission to the IMW, and therefore not included in this study.
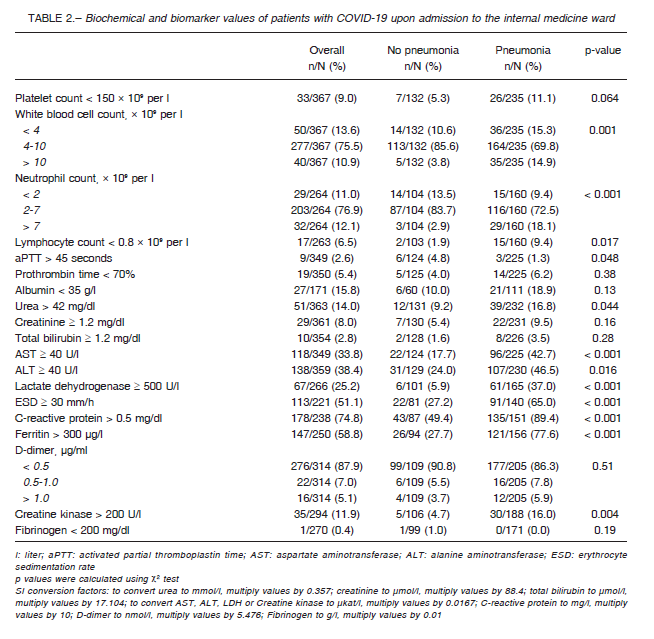
Baseline laboratory findings as categorical variables can be observed in Table 2. Upon admission, most patients had normal white blood cell (WBC) count. However, patients with pneumonia exhibited higher proportion of both lower and higher WBC counts, and lymphopenia was more common (Table 2). Most patients had elevated C-reactive protein and ferritin levels regardless of pneumonia diagnosis, though a higher proportion of pneumonia patients had elevated serum values of this biomarkers together with erythrocyte sedimentation rate and creatine kinase.
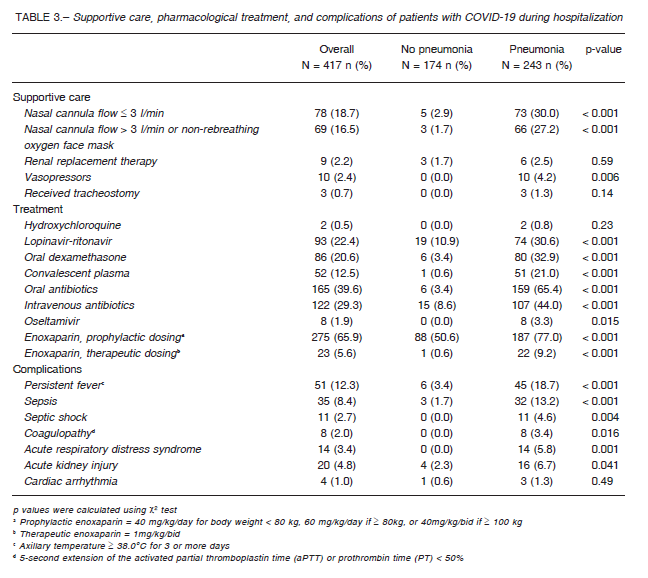
More than half of COVID-19 with pneumonia required oxygen supplementation during hospitalization (Table 3).
Although nine underwent hemodialysis during their inpatient stay, six of them were already in renal replacement therapy whereas in the other three patients it was required because of AKI.
Four types of pharmacological alternatives for the treatment of COVID-19 were available in our center at different moments during the epidemic: hydroxychloroquine (HCQ), lopinavir-ritonavir (LPV/r), convalescent plasma transfusion (CPT), and low-dose oral dexamethasone (Table 3). HCQ was administered to only two patients before compassionate use was discontinued at our center and LPV/r became unavailable in June 2020. Antibiotics were used in only a few patients without pneumonia, but 234 (96.3%) with COVID-19 pneumonia received oral or intravenous antibiotics during their stay. A higher proportion of complications was observed among those with pneumonia (Table 3).
None of the COVID-19 without pneumonia developed critical disease, required IMV nor died during hospitalization and their median hospital stay was shorter (Table 4). Of these patients, 13 (7.5%) were deemed to have pneumonia on further diagnostic evaluation after 48hs from admission and seven of them developed severe disease during hospitalization. A higher incidence
of severe and critical disease was observed among patients with pneumonia. However, most of them had mild disease during hospitalization and were discharged alive (Table 4).
Discussion
In order to describe the clinical characteristics and outcomes of COVID-19 with and without pneumonia, this prospective cohort study included 417 non-critically ill COVID-19 patients admitted to the internal medicine ward (IMW) of a public hospital in Buenos Aires, Argentina.
As expected, COVID-19 patients with no evidence of pneumonia had a largely good prognosis, with more than 95% of them safely discharged after a short inpatient stay.
None of them developed critical disease, required ICU admission nor died during hospitalization. Still, it is important to note that 4% of these patients developed severe disease requiring oxygen supplementation. Presently, home care of COVID-19 patients is a well-established management strategy and guidelines for its implementations has been published by the World Health Organization (WHO)18. As the understanding of the disease evolved and the development of extra-hospital facilities consolidated, outpatient management of COVID-19 became a mainstream approach to ameliorate the burden imposed on the IMWs throughout the city.
Our results support the outpatient management of COVID-19 patients with mild disease, if proper isolation can be ensured, as systematic hospitalization of these patients seems disproportionate to the low incidence of complications, especially in the context of overwhelmed health systems. However, discharged patients should be warned of early signs of disease progression and, appropriate means for monitoring the clinical course of patients with COVID-19 at home should be assured, given that unfavorable progression can take place even after determining mild disease in a comprehensive initial evaluation.
In contrast, severe and critical disease developed in one-third and 10% of patients with COVID-19 pneumonia, respectively, and 9.1% were admitted to the ICU. Among different cohort studies, there is great heterogenicity in the reported rates of ICU admission, mostly from 20% to 30%14, 19-23. However, some studies have reported a frequency lower than 10%24-26. Careful consideration must be given to comparisons of ICU admission rates given that differences could be explained by different methodologies or demographic characteristics of the study populations (i.e.: younger cohort age), rather than differences in the clinical features of the disease or quality, quantity, and capacity of healthcare facilities.
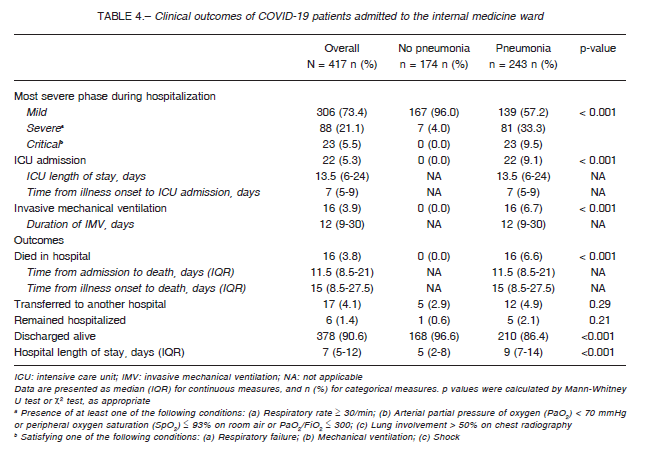
In-hospital mortality for patients with COVID-19 pneumonia in our cohort was 6.6% and crude case-fatality rate for all COVID- 19 admitted to the IMW regardless of pneumonia diagnosis was 3.8%. A lower lethality rate (1.8%) was observed in the epidemiological report of the first 116974 cases in Argentina, consistent with the lower median age and comorbidities provided the population-based nature of the report as opposed to our cohort of inpatients27. Broad variations in estimations of case-fatality rates have been observed among countries, ranging from less than 1% (South Korea, Germany) to almost 9% (Italy)28. Argentina rates rank among the lowest in the world, though, many factors influence the variability among mortality rates and narrow interpretations can be misleading, as reported by the WHO29.
Upon admission, almost 60% of the study population had pneumonia and many demographic and clinical differences were found between patients with and without pneumonia. Those with pneumonia were older and a higher prevalence of males, obesity, diabetes, hypertension, and history of neoplastic disease was observed.
However, careful consideration should be given when pondering if these features confer a higher risk of developing pneumonia in patients with SARS-CoV-2, given the non-randomized nature of this study.
In our cohort, diabetes was strongly associated with pneumonia diagnosis, with a 3.5-fold higher prevalence of this condition among patients with COVID-19 pneumonia as compared to those without. An increased risk of infections in individuals with diabetes has been described and a comprehensive review of the pathological mechanisms of this association in COVID-19 is examined in a recent article by Bornstein et al30. Hence, our results contribute to the increasingly body of evidence linking diabetes to an increased risk of developing pulmonary lesions following SARS-CoV-2 infection.
Obesity was twice as prevalent in patients with COVID-19 pneumonia vs. patients in which pneumonia was ruled out upon admission to the IMW. There is sound evidence connecting obesity to COVID-19 adverse outcomes, in both higher risk of developing the infection itself as well as an increased rate of hospitalization, ICU admission and mortality, especially in older people and patients with other comorbidities31-33. Underlaying mechanisms for this associations include both immune and metabolic derangement and have been reviewed elsewhere34, 35.
A higher prevalence of hypertension was observed compared to patients without pneumonia. However, this finding does not imply a causal relationship since hypertension is more frequent in older and associated to other prevalent comorbidities that may also contribute to a higher risk of developing pneumonia after infection with SARS-CoV-236.
A short duration of illness before admission was observed in this study, probably because the public health recommendation in Argentina was to seek immediate care even with mild disease clinical presentation37. It is important to note that this study considers pneumonia diagnosis within 48 h of admission to the hospital. Consequently, most patients in this cohort were assessed at the initial stages of the disease when radiological evidence of pneumonia may have been absent. Some patients without pneumonia on admission developed pneumonia at some point during follow-up but a systematic assessment of new lung infiltrates after admission was out of the scope of this study. Still, clinical deterioration was thoroughly assessed as part of the study outcomes and therefore, it is important to reinforce the need for periodic evaluations of COVID-19 patients even in those presenting with mild disease.
The present study has some limitations. Firstly, pneumonia diagnosis for this study is based only on chest x-ray results or positive findings on physical examination, since computed tomography (CT) was not systematically performed on admitted patients. As CT is more sensitive for early parenchymal lung disease38, 39, some patients categorized as without pneumonia would have shown infiltrates on CT had it been performed, leading to underreporting of COVID-19 pneumonia in asymptomatic patients. Secondly, patients admitted to the ICU without prior hospitalization into the IMW (i.e.: straight from the emergency department) are not included. Therefore, our results cannot be made extensive to all hospitalized during the study period. However, our cohort adequately captures the clinical course of COVID-19 inpatients without critical disease upon admission. Thirdly, routinely collected data is prone to missing data and biases. Still, the prospective nature of the study allowed for the recovery of most of the data and outcome assessment was made using the study’s definitions and not solely on the diagnoses included in medical records. Finally, in Buenos Aires City, COVID-19 patients are treated in numerous public and private institutions. As this is a single-center study conducted in a public hospital, results will not be representative of all patients in Buenos Aires. Despite these limitations, our center constitutes a referral hospital for COVID-19 within the public health system of the City of Buenos Aires and to the best of our knowledge, this is the largest prospective study among patients with COVID-19 in Argentina.
Our results contribute to the growing body of evidence from Latin American countries and will surely advance the understanding of the clinical characteristics of COVID-19.
In conclusion, a largely good prognosis was observed among COVID-19 patients without pneumonia, still, even among this group, unfavorable clinical progression can develop and should be properly monitored. Critical illness among patients with COVID-19 pneumonia was frequent and observed rates from this cohort provide a comprehensive characterization of clinical outcomes among patients admitted to an internal medicine ward.
Acknowledgments: The authors wish to thank all the staff members of the Internal Medicine Unit. Their invaluable work in the internal medicine wards has been pivotal in the management of the pandemic
Conflict of interest: None to declare
References
1. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med 2020; 382: 1199-207.
2. Gemelli NA. Management of COVID-19 Outbreak in Argentina: The beginning. Disaster Med Public Health Prep 2020: 1-3. doi: 10.1017/dmp.2020.116
3. Ministerio de Salud de la Argentina. Evaluación inicial del paciente con infección respiratoria aguda y decisión del sitio de internación. In: https: //www.argentina.gob.ar/salud/coronavirus-COVID-19/evaluacion-inicial-ira; accessed July 2020
4. Centers for Disease Control and Prevention (CDC). Interim Guidance: Home Care for 2019-nCoV. In: https: //www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-home-care. html; accessed July 2020
5. Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect 2020; 81: e16-e25.
6. Tian W, Jiang W, Yao J, et al. Predictors of mortality in hospitalized COVID-19 patients: A systematic review and meta-analysis. J Med Virol 2020; 92: 1875-83.
7. Lopardo G, Basombrío A, Clara L, Desse J, De Vedia L. Neumonía adquirida de la comunidad en adultos. Recomendaciones sobre su atención. Medicina (B Aires) 2015; 75: 245-57.
8. The Global Health Network. Clinical Data Collection – The COVID-19 Case Report Forms (CRFs). In: https: //isaric.tghn.org/COVID-19-CRF/; accessed May 2020.
9. Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med 2020. doi: 10.1056/NEJMcp2009575. Online ahead of print.
10. Charlson ME, Pompei P, Ales KL, MacKenzie R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373-83.
11. Lim WS, Van Der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax 2003; 58: 377-82.
12. Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsisrelated Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med 1996; 22: 707-10.
13. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. Lancet 1974; 304: 81-4.
14. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054-62.
15. Rahman M. Acute kidney injury: a guide to diagnosis and management. Am Fam Physician 2012; 86: 631-9.
16. Melendi SE, Pérez M, Salas C, et al. Estudio de cohorte prospectivo de pacientes con COVID-19 hospitalizados en el Servicio de Clínica Médica del Hospital Durand: protocolo de estudio. Rev Argent Salud Pública 2020; 12(Supl COVID-19): e7.
17. von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453-7.
18. World Health Organization. Home care for patients with suspected or confirmed COVID-19 and management of their contacts. In: https: //www.who.int/publications/i/item/home-care-for-patients-with-suspected-novel-coronavirus-(ncov)-infection-presenting-with-mild-symptoms-and-management-of-contacts; acceased September 2020.
19. Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis 2020; 34: 101623.
20. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020; 323: 1061-9.
21. Centers for Disease Control and Prevention (CDC). Management of patients with confirmed 2019-nCoV | CDC. In: https: //www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html; accessed September 2020.
22. Pellaud C, Grandmaison G, Pham Huu Thien HP, et al. Characteristics, comorbidities, 30-day outcome and inhospital mortality of patients hospitalised with COVID-19 in a Swiss area – a retrospective cohort study. Swiss Med Wkly 2020; 150: w20314.
23. Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ 2020; 369. doi: 10.1136/bmj.m1966.
24. Livingston E, Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. JAMA 2020; 323: 1335-5.
25. Montagnani A, Pieralli F, Gnerre P, Vertulli C, Manfellotto D. COVID-19 pandemic and Internal Medicine Units in Italy: a precious effort on the front line. Intern Emerg Med 2020: 1-3. doi: 10.1007/s11739-020-02454-5.
26. Guan W, Ni Z, Hu YY, et al. Clinical characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020; 382: 1708-20.
27. Rearte A, Baldani AEM, Barcena Barbeira P, et al. Características epidemiológicas de los primeros 116 974 casos de covid-19 en Argentina, 2020. Rev Argent Salud Pública 2020; 12(Supl COVID-19).
28. Lau H, Khosrawipour T, Kocbach P, Ichii H, Bania J, Khosrawipour V. Evaluating the massive underreporting and undertesting of COVID-19 cases in multiple global epicenters. Pulmonology 2020. doi: 10.1016/j.pulmoe.2020.05.015.
29. World Health Organization. Estimating mortality from COVID-19. In: https: //www.who.int/news-room/commentaries/detail/estimating-mortality-from-covid-19; accessed September 2020.
30. Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol 2020; 8: 546-50.
31. Popkin BM, Du S, Green WD, et al. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes Rev 2020; 21(11). doi: 10.1111/obr.13128.
32. de Siqueira JVV, Almeida LG, Zica BO, Brum IB, Barceló A, de Siqueira Galil AG. Impact of obesity on hospitalizations and mortality, due to COVID-19: A systematic review. Obes Res Clin Prac 2020; 14: 398-403.
33. Hussain A, Mahawar K, Xia Z, Yang W, EL-Hasani S. Obesity and mortality of COVID-19. Meta-analysis. Obes Res Clin Pract 2020; 14: 295-300.
34. Ritter A, Kreis NN, Louwen F, Yuan J. Obesity and COVID-19: Molecular mechanisms linking both pandemics. Int J Mol Sci 2020; 21(16). doi: 10.3390/ijms21165793.
35. Korakas E, Ikonomidis I, Kousathana F, et al. Obesity and COVID-19: Immune and metabolic derangement as a possible link to adverse clinical outcomes. Am J Physiol Endocrinol Metab 2020; 319: E105-E109.
36. Tadic M, Cuspidi C, Grassi G, Mancia G. COVID-19 and arterial hypertension: Hypothesis or evidence? J Clin Hypertens 2020; 22: 1120-6.
37. Ministerio de Salud de la Argentina. Definición de caso. In: https: //www.argentina.gob.ar/salud/coronavirus-COVID-19/definicion-de-caso; accessed November 2020.
38. Rubin GD, Ryerson CJ, Haramati LB, et al. The role of chest imaging in patient management during the COVID-19 pandemic: A Multinational Consensus Statement From the Fleischner Society. Chest 2020; 158: 106-16.
39. Li H-W, Zhuo L-H, Yan G-W, et al. High resolution computed tomography for the diagnosis of 2019 novel coronavirus (2019-nCoV) pneumonia: a study from multiple medical centers in western China. Ann Transl Med 2020; 8: 1158.
