VANESA ZYLBERMAN 1, 2, SANTIAGO SANGUINETI 1, ANDREA V. PONTORIERO 3, SANDRA V. HIGA 4, MARÍA L. CERUTTI 2, 5, SUSANA M. MORRONE SEIJO 1, ROMINA PARDO 1, LUCIANA MUÑOZ 1, MARÍA E. ACUÑA INTRIERI 2, 5, VANINA A. ALZOGARAY 6, MARTÍN M. AVARO 3, ESTEFANÍA BENEDETTI 3, PAULA M. BERGUER 6, LAURA BOCANERA 7, LUCAS BUKATA 1, MARINA S. BUSTELO 1, ANA M. CAMPOS 3, MARIANA COLONNA 1, ELISA CORREA 7, LUCÍA CRAGNAZ 7, MARÍA E. DATTERO 3, MARÍA DELLAFIORE 7, SABRINA FOSCALDI 6, JOAQUÍN V. GONZÁLEZ 1, LUCIANO L. GUERRA 7, SEBASTIÁN KLINKE 6, MARÍA S. LABANDA 6, CONSTANZA LAUCHÉ 1, JUAN C. LÓPEZ 4, ANABELA M. MARTÍNEZ 4, LISANDRO H. OTERO 6, ELÍAS H. PEYRIC 4, PABLO F. PONZIANI 4, ROMINA RAMONDINO 1, JIMENA RINALDI 6, SANTIAGO RODRÍGUEZ 7, JAVIER E. RUSSO 4, MARA L. RUSSO 3, SOLEDAD L. SAAVEDRA 4, MAURICIO SEIGELCHIFER 7, SANTIAGO SOSA 6, CLAUDIO VILARIÑO 5, PATRICIA LÓPEZ BISCAYART 4, ESTEBAN CORLEY 7, LINUS SPATZ 1, ELSA G. BAUMEISTER 3*, FERNANDO A. GOLDBAUM 1, 5, 6*
1 Inmunova S.A., San Martín, Provincia de Buenos Aires, 2 Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), 3 Servicio Virosis Respiratorias INEI-ANLIS Malbrán, Laboratorio Nacional de Referencia de Enfermedades Respiratorias Virales, Centro Nacional de Influenza de OMS, Buenos Aires, 4 Instituto Biológico Argentino S.A.I.C., Buenos Aires, 5 CRIP -Centro de Rediseño e Ingeniería de Proteínas UNSAM, Campus Miguelete, San Martín, Provincia de Buenos Aires, 6 Laboratorio de Inmunología y Microbiología Molecular, Fundación Instituto Leloir, IIBBA-CONICET, Buenos Aires, 7 mAbxience, Munro, Provincia de Buenos Aires, Argentina
Resumen La enfermedad denominada COVID-19 es causada por el coronavirus SARS-CoV-2 y es actualmente considerada una pandemia a nivel global. El desarrollo de vacunas es sin duda la mejor estrategia a largo plazo, pero debido a la emergencia sanitaria, existe una necesidad urgente de encontrar soluciones rápidas y efectivas para el tratamiento de la enfermedad. Hasta la fecha, el uso de plasma de convalecientes es la única inmunoterapia disponible para pacientes hospitalizados con COVID-19. El uso de anticuerpos policlonales equinos (EpAbs) es otra alternativa terapéutica interesante. La nueva generación de EpAbs incluyen el procesamiento y purificación de los mismos y la obtención de fragmentos F(ab’)2 con alta pureza y un excelente perfil de seguridad en humanos. Los EpAbs son fáciles de producir, lo cual permite el desarrollo rápido y la elaboración a gran escala de un producto terapéutico. En este trabajo mostramos el desarrollo de un suero terapéutico obtenido luego de la inmunización de caballos utilizando el receptor-binding domain de la glicoproteína Spike del virus. Nuestro producto mostró ser alrededor de 50 veces más potente en ensayos de seroneutralización in vitro que el promedio de los plasmas de convalecientes. Estos resultados nos permitirían testear la seguridad y eficacia de nuestro producto en ensayos clínicos de fase 2/3 a realizarse a partir de julio de 2020 en la zona metropolitana de Buenos Aires, Argentina.
Palabras clave: COVID-19, Argentina, suero equino hiperinmune, fragmentos F(ab’)2
Abstract The disease named COVID-19, caused by the SARS-CoV-2 coronavirus, is currently generating a global pandemic. Vaccine development is no doubt the best long-term immunological approach, but in the current epidemiologic and health emergency there is a need for rapid and effective solutions. Convalescent plasma is the only antibody-based therapy available for COVID-19 patients to date. Equine polyclonal antibodies (EpAbs) put forward a sound alternative. The new generation of processed and purified EpAbs containing highly purified F(ab’)2 fragments demonstrated to be safe and well tolerated. EpAbs are easy to manufacture allowing a fast development and scaling up for a treatment. Based on these ideas, we present a new therapeutic product obtained after immunization of horses with the receptor-binding domain of the viral Spike glycoprotein. Our product shows around 50 times more potency in in vitro seroneutralization assays than the average of convalescent plasma. This result may allow us to test the safety and efficacy of this product in a phase 2/3 clinical trial to be conducted in July 2020 in the metropolitan area of Buenos Aires, Argentina.
Key words: COVID-19, Argentina, hyperimmune horse sera, F(ab’)2 fragments
Postal address: Fernando A. Goldbaum, Inmunova S.A., 25 de Mayo 1021, 1650 San Martín, Provincia de Buenos Aires, Argentina
e-mail: fgoldbaum@inmunova.com
Postal address: Elsa G. Baumeister, Servicio Virosis Respiratorias INEI-ANLIS Malbrán, Laboratorio Nacional de Referencia de Enfermedades Respiratorias Virales, Centro Nacional de Influenza de OMS, Buenos Aires, Argentina
e-mail: ebaumeister@anlis.gob.ar
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), is currently generating a global pandemic with more than 9.3 million infections and 478 000 fatalities (as of June 23rd, 2020) (https://www.worldometers.info/coronavirus/) 1. In Argentina, despite the early quarantine introduced by the Argentine government, SARS-CoV-2 has caused more than 44 300 infections and around 1040 deaths. Immunotherapy appears as the most promising therapeutic approach to alleviate the burden of this disease. Convalescent plasma is the only antibody-based therapy available for COVID-19 patients to date. This strategy has proved to be safe and preliminary studies showed that plasma transfusion is a potentially efficient treatment option for patients hospitalized with COVID-19. However, it is not clear whether enough donors with adequate levels of antibodies will be available for a massive application of this approach.
Vaccine development is no doubt the best long-term immunological approach, but in the current epidemiological and health emergency there is a need for rapid and effective solutions. Equine polyclonal antibodies (EpAbs) can be one of these interim solutions. EpAbs recognize a vast array of epitopes (limiting the risk of viral escape mutations) and tend to develop greater avidity than monoclonal antibodies (mAbs) for their cognate antigens. In addition, EpAbs are easy to manufacture allowing a fast development and scaling up for human use. While in the past the risk of serum sickness −mainly due to the presence of Fc fragments−disfavored the use of EpAbs, the new generation of processed and purified EpAbs containing highly purified F(ab’)2 fragments demonstrated to be safe and well tolerated. This kind of products has been used for decades in the management of clinical emergencies, such as snakebite and scorpion sting envenomation, severe poisoning (tetanus toxin, digoxin and, more recently, botulinum toxin) and severe infectious diseases like avian influenza. Starting in 2015, the Argentine biotech startup company Inmunova has developed a therapy based on Neutralizing Equine Anti Shiga Toxin (NEAST) F(ab´)2 fragment antibodies for the prevention of the hemolytic-uremic syndrome 2. The NEAST therapy has successfully passed preclinical and phase 1 analysis, showing an adequate profile of safety and pharmacokinetics (unpublished results). A phase 1 final report was submitted to the Argentinean health authority Agency (ANMAT) and its favorable evaluation enabled the initiation of a phase 2/3 clinical trial in the pediatric population, which is currently ongoing (http://www.clinicaltrials.gov, identifier NCT04132375).
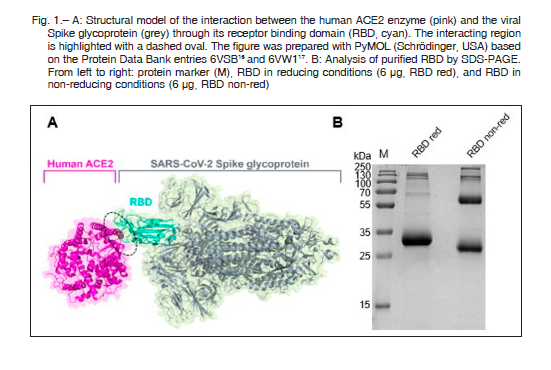
A recent report has shown that the receptor binding domain (RBD) from the viral Spike glycoprotein elicits high titers of neutralizing antibodies against SARS-CoV-2 when used as an immunogen in horses 3. Based on this study, and in the previous experience of Inmunova, at the beginning of April 2020 we launched a protocol to produce equine hyperimmune sera for the treatment of COVID-19 patients. In this article, we describe the rapid and efficient production of therapeutic hyperimmune sera from which we obtained the F(ab´)2 fragment products that will be entering clinical trials in the next weeks. For this purpose, we also selected the RBD domain of the SARS-CoV-2 Spike protein as an immunogen 3, 4 since several recent reports indicate that most of the Spike neutralizing epitopes reside in this domain 5, 6. Structural biology analysis has explained this fact at the molecular level. As shown in Figure 1A, the contact of the virus with its cellular human receptor, the angiotensin converting enzyme 2 (ACE2), is mediated by a strong protein-protein interaction between the enzyme and the RBD. Thus, antibodies directed against the RBD surface are thought to block the viral entry to host cells.
Recent publications also demonstrated that sera from SARS-CoV-2 infected patients show a clear correlation between reactivity in enzyme-linked immunosorbent assays (ELISA) against RBD and titers obtained in seroneutralization assays 7, 8. In addition, the use of RBD as an immunogen avoids eliciting antibodies against other domains of Spike that could have deleterious effects, such as antibody dependent enhancement 9, 10.
Recombinant RBD was produced using the mammalian expression plasmid pCAGGS, which was kindly provided by Prof. Florian Krammer 4. This plasmid contains the coding region for RBD (residues 319 to 541 of Spike) along with a signal peptide (residues 1-14) and a C-terminal hexahistidine tag. The plasmid was transfected in human embryonic kidney (HEK) 293T cells (ATCC CRL-3216, USA). In brief, cells were grown at 37°C with 5% CO2 in Dulbecco’s Modified Eagle medium (DMEM, Gibco, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS, Gibco), 100 U/ml penicillin, 100 μg/ml streptomycin and 0.25 μg/ml of amphotericin B. For transfection, cells with 80-90% confluency in 100 mm tissue culture plates were transfected with purified plasmid DNA using polyethylenimine (PEI 87 kDa, Facultad de Farmacia y Bioquímica FFyB-UBA, Argentina) in serum-free media.
The supernatants from transfected cells were harvested on day 3-4 post-transfection by centrifugation, and soluble RBD was purified by affinity chromatography on a HisTrap HP column (GE Healthcare, UK) following standard procedures.
The eluted pool was dialyzed overnight against high salt (300 mM sodium chloride) PBS and concentrated to 0.8-1.1 mg/ml. Then, the protein was sterilized by filtration and stored at 2-8 °C until use. We reached a yield of about 7.5 mg per liter of culture, as estimated by measuring its absorbance at 280 nm and by reducing SDS-PAGE stained with Coomassie blue. The purity of the recombinant protein was checked by SDS-PAGE in both reducing and non-reducing conditions (Fig. 1B) and by analytical size exclusion chromatography (data not shown). A single predominant band was observed at approximately 32 kDa under reducing conditions (corresponding to the expected molecular weight of the protein [26 kDa] plus glycosylation). The presence of RBD dimers under non-reducing conditions is worth to mention 3, 11.
A previous work showed that recombinant RBD can elicit strong reactivity and seroneutralization activities in mice and horses 3. We designed our immunization protocol based on this study but using a smaller amount of RBD.
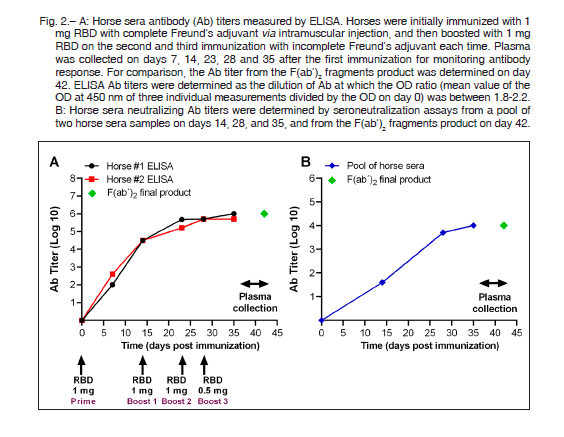
Explicitly, we used approximately ten times less protein than in the referred work, and obtained similar or even higher responses, as measured by ELISA using RBD as an antigen (Fig. 2A).
In order to measure the antibody response elicited by the immunized horses at different times post immunization, we performed an indirect ELISA using purified RBD as an antigen. In brief, 96-well plates (Nunc, Merck, USA) were coated with 0.25 μg per well of RBD in PBS overnight at 2-8 °C. The blocking solution was 3% non-fat milk prepared in PBS with 0.1% Tween 20 (TPBS). To calculate the horse serum titers, two-fold serial dilutions of horse serum samples prepared in 1% non-fat milk in TPBS were added to the plates and incubated at 37 °C for 1 h. Horseradish peroxidase-conjugated goat anti-horse F(ab´)2
(Sigma, Germany) diluted 10 000-fold and substrate tetramethylbenzidine solution (Abcam, UK) were used for detection. Values of absorbance at 450 nm greater than twice those of the negative controls were considered as positive. As shown in Figure 2A, we obtained strong antibody responses using 1 mg of RBD for each of three repeated immunizations (on days 0, 14 and 23) and 0.5 mg for the final boost (on day 28), compared with 3, 6 and 12 mg used in the previous report 3.
In parallel with the ELISA assay, we analyzed the in vitro neutralization capacity of horse sera using a seroneutralization assay against SARS-CoV-2. In brief, a SARS-CoV-2 strain was isolated from a clinical specimen of a COVID-19 confirmed Argentinean patient. For the assay, Vero cells were seeded in 96-well plates with 1.5 × 104 cells/well overnight. Horse sera were firstly diluted in 2% fetal bovine serum-Dulbecco’s modified Eagle’s medium supplemented with 1600 U/ml of penicillin, 800 μg/ml of streptomycin and 10 μg/ml of amphotericin B and incubated with 1000 TCID50 (the tissue culture infective dose) of SARS-CoV-2 at 37°C for 1 h. The mixture was then added to the cells and incubated at 37 °C for 1 h. The cells were washed with PBS and incubated in the same medium as before. The cytopathic effect was examined for 3 days post-infection. The complete absence of cytopathic effect in an individual culture well was defined as protection. This assay showed that horse hyperimmune sera reached very high neutralizing titers after the second immunization, with values of 1:10 240 that were remarkably conserved after the manufacture of the final product, as explained below (Fig. 2B).
As the ELISA reactivity reached a plateau after the third immunization with a high neutralizing potency, we decided to collect plasma from two horses at day 34 of the immunization plan. In order to obtain the first clinical batch, the plasma was digested with pepsin under controlled conditions rendering F(ab´)2 fragments from the immunoglobulin molecules. The fragments were then further purified by salting out and membrane Q chromatography.
The bulk solution was nanofiltered (20 nm), then sterilized through a 0.2 μm pore cartridge and filled in sterile glass vials. The total protein content in the final product was adjusted to 30 mg/ml. The first batch was released using the same good manufacturing practice standard used for other equine hyperimmune sera produced at the Instituto Biológico Argentino.
As previously mentioned, a similar strategy was followed during the production of NEAST, which demonstrated to be a safe product. From a regulatory point of view, health authorities might consider that the obtained anti-SARS-CoV-2 F(ab´)2 antisera should be similar to NEAST, since both products only differ in their specificities.
Besides, the safety of this type of products has been extensively demonstrated 12. Thus, it can be considered that, as both products were manufactured using the same platform and have similar compositions, under the circumstances of a pandemic, the anti-SARS-CoV-2 F(ab´)2 antisera can advance promptly to a phase 2/3 clinical trial, allowing the rapid use of this product during this emergency.
Notably, the results indicated that our product has a high neutralizing capacity against SARS-CoV-2, as previously observed 3. Recent studies on the potential therapeutic use of convalescent human plasma indicate highly variable neutralizing responses among individuals, with an average geometric mean of 1:200 3, 8, 13, 14. Thus, with titers of 1:10 240, the obtained horse hyperimmune sera bear around 50 times stronger neutralizing capacity than the average neutralizing capacity shown by convalescent plasma.
Since neutralizing antibodies should block the internalization of SARS-CoV-2 to lung cells, it has been postulated that their passive administration should render maximal clinical benefits if they are applied at early stages of the disease. Several clinical studies suggest that convalescent plasma therapy applied to critical patients in intensive care unit have only limited effects 7, 15. However, the limited availability of convalescent plasma, turns the application of this therapy to more patients at early stages a real challenge.
Since EpAbs do not have this limitation, our clinical plan is to apply our hyperimmune serum to patients with moderate to severe disease according to the published COVID-19 Treatment Guidelines (https://covid19treatmentguidelines.nih.gov/) with less than 10 days from the onset of symptoms. We anticipate that the clinical trial might begin at early July 2020. Should this clinical trial evidence the efficacy of the therapy by improving patient outcome, we have the potential to scale up the anti-SARS-CoV-2 hyperimmune serum production for extensive clinical application in the current epidemiological situation.
In conclusion, in this work we show that RBD-immunized horses elicit a large amount of anti-SARS-CoV-2 neutralizing antibodies, which are capable of neutralizing in vitro the virus with around 50 times more potency than the average neutralizing capacity shown by convalescent plasma. We have produced F(ab´)2 polyclonal antibodies that proved to have equally high neutralizing capacity and will test them in the next weeks for their efficacy as passive immunotherapy in patients undergoing early stages of the disease. Besides, the safety profile of this type of sera is well known and our antibodies are devoid of the Fc domain, which averts potential triggering of antibody dependent enhancement or cytokine storms. All of this reinforces the idea that our therapeutic strategy will play a preponderant role in COVID-19 management until an effective vaccine is developed.
Acknowledgements: We want to especially acknowledge the continuous support of Grupo Insud to the work of Inmunova. This work was partially supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), the Ministerio de Ciencia, Tecnología e Innovación (MINCyT), and the Agencia Nacional de Promoción de la Investigación, el Desarrollo Tecnológico y la Innovación, under the Coronavirus Unit Initiative (Grant No. IP-COVID-19 #609-746). We are grateful to Andrea Gamarnik and coworkers at the Fundación Instituto Leloir working under the Coronavirus Unit Initiative of MINCyT for their assistance in the production of RBD. We are also indebted to Claudia Perandones (Technical Scientific Director, ANLIS Malbrán, Ministerio de Salud de Argentina) and Viviana Molina (Director of the Instituto Nacional de Enfermedades Infecciosas – ANLIS Malbrán) for their help in coordinating activities. We acknowledge the support of the authorities of the Ministerio de Salud de Argentina, Universidad Nacional de General San Martín and Fundación Argentina de Nanotecnología. We especially thank all the administrative and technical staff of Inmunova (C.D., C.L., F.C., L.M., L.Z.) for their great work under very difficult circumstances.
Conflicts of interest: None to declare
References
1. COVID-19 Coronavirus Pandemic Situation. Last updated June 23, 2020. In: https://www.worldometers.info/coronavirus/; accessed June 2020.
2. Hiriart Y, Pardo R, Bukata L, et al. Desarrollo de un producto anti-toxina Shiga para la prevención del síndrome urémico hemolítico. Medicina (B Aires) 2018; 78: 107-12.
3. Pan X, Zhou P, Fan T, et al. Immunoglobulin fragment F(ab’)2 against RBD potently neutralizes SARS-CoV-2 in vitro. bioRxiv 2020. doi: https://doi.org/10.1101/2020.04.07.029884.
4. Amanat F, Stadlbauer D, Strohmeier S, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med 2020. doi: 10.1038/s41591-020-0913-5. Online ahead of print.
5. Wang C, Li W, Drabek D, et al. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun 2020; 11: 2251.
6. Wu Y, Wang F, Shen C, et al. A noncompeting pair of human neutralizing antibodies block COVID-19 virus binding to its receptor ACE2. Science 2020; 368: 1274-8.
7. Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA 2020; e2010044. Online ahead of print.
8. Ni L, YeF, Cheng M-L, et al. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity 2020; 52: 971-7.e3.
9. Quinlan BD, Mou H, Zhang L, et al. The SARS-CoV-2 receptor-binding domain elicits a potent neutralizing response without antibody-dependent enhancement. bioRxiv 2020. doi: https://doi.org/10.1101/2020.04.10.036418.
10. Wang J, Zand MS. The potential for antibody-dependent enhancement of SARS-CoV-2 infection: Translational implications for vaccine development. J Clin Transl Sci 2020; 1-4. doi: 10.1017/cts.2020.39. Online ahead of print.
11. Chen WH, Chag SM, Poongavanam MV, et al. Optimization of the production process and characterization of the yeast-expressed SARS-CoV recombinant receptor-binding domain (RBD219-N1), a SARS vaccine candidate. J Pharm Sci 2017; 106: 1961-70.
12. Boyer L, Degan J, Ruha A-M, Mallie J, Mangin E, Alagón A. Safety of intravenous equine F(ab’)2: Insights following clinical trials involving 1534 recipients of scorpion antivenom. Toxicon 2013; 76: 386-93.
13. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA 2020; 323: 1582-9.
14. Zhang B, Liu S, Tan T, et al. Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest 2020; S0012-3692(20)30571-7. Online ahead of print.
15. McAllister F, Mantegazza A, Garzón F, et al. Uso de plasma de convalecientes para tratamiento de COVID-19: Historia y evidencia. Medicina (B Aires) 2020. In: https://www.medicinabuenosaires.com/revistas/vol80-20/destacado/editorial_7213.pdf; accessed June 2020.
16. Wrapp D, Wang N, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020; 367: 1260-3.
17. Shang J, Ye G, Shi K, et al. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020; 581: 221-4.
