ADRIANA VARÓN 1, 2, LUISA SANTOS 1, MÓNICA TAPIAS 3, 4, CLARA CÁEZ 5, JUAN IGNACIO MARÍN 6, 7, ÓSCAR SANTOS 6, MARTÍN GARZÓN 1, 2, ÓSCAR BELTRÁN 1, 2, ANDRÉS GÓMEZ-ALDANA 3, 8, ISMAEL J. YEPES 9, MARTÍN RONDÓN 10, DIEGO ROSSELLI 10
1 Servicio de Hepatología y trasplante hepático, Fundación Cardioinfantil, Bogotá, Colombia, 2 Universidad del Rosario, Bogotá, Colombia, 3 Hospital Universitario Fundación Santa Fe de Bogotá, Bogotá, Colombia, 4 Organización Sanitas Internacional, Bogotá, Colombia, 5 Servicio de Hepatología, Barranquilla, Colombia, 6 Grupo de Hepatología y Trasplante Hepático, Hospital Pablo Tobón Uribe, Medellín, Colombia, 7 Universidad Pontificia Bolivariana, Medellín, Colombia, 8 Gut Médica, Bogotá, Colombia, 9 Pharos Grupo de investigación Ciencia, Tecnología y Salud, Universidad de Cartagena, Cartagena, Colombia, 10 Departamento de Epidemiología y Bioestadística Clínica, Facultad de Medicina, Pontificia Universidad Javeriana, Bogotá, Colombia
Abstract There are few published real-world studies on hepatitis C in Latin America. This paper describes a cohort of Colombian subjects treated with direct-acting antiviral agents. A total of 195 patients from 5 hepatology centers in 4 Colombian cities were retrospectively studied. For each patient, serum biomarkers were obtained, and Child-Pugh, MELD, cirrhosis and fibrosis stage were calculated. Additionally, viral load was quantified at initiation, end of treatment and at 12 weeks of completion. Adverse effects were recorded. Patients with liver transplant were compared with non-transplanted patients in terms of serum biomarkers. The patients had received 9 different regimes. The most prevalent viral genotype was 1b (81.5%). Overall, 186 patients (95.4%) attained sustained virologic response. When comparing transplanted vs. non-transplanted patients, those in the non-transplanted group were more likely to have cirrhosis (52.6% vs. 12.5%, p = 0.0004). Pre-treatment viral load was higher in the transplant group (1 743 575 IQR = 1 038 062-4 252 719 vs. 345 769 IQR = 125 806-842 239; p < 0.0001) as well as ALT and AST levels (82.5 IQR 43.5-115.5 vs. 37.0 IQR = 24.7-73.3; p = 0.0009 and 70 IQR = 41-140 vs. 37 IQR = 24-68; p = 0.004 respectively). Adverse events were reported by 28.7% of the patients; asthenia (5.6%) was the most prevalent. Our results are comparable with those from other countries in terms of therapy and biomarkers. However, our cohort reported less adverse events. Further research is needed in the region.
Key words: antiviral agents, drug therapy, hepatitis C, chronic hepatitis, liver transplantation, treatment outcome
Resumen Experiencia colombiana en el tratamiento de la hepatitis C con agentes antivirales de acción directa. Existen pocas publicaciones de evidencias del mundo real sobre hepatitis C en América Latina. En este estudio presentamos una cohorte colombiana de pacientes tratados con agentes antivirales de acción directa. Fueron analizados retrospectivamente 195 pacientes seleccionados en 5 centros de hepatología en 4 ciudades de Colombia. Dos tercios fueron mujeres y la mitad tenía ≥ 62 años. De cada uno se cuantificaron biomarcadores séricos, escala de Child-Pugh, MELD y grado de cirrosis y fibrosis. Se cuantificó carga viral al inicio, al final y a las 12 semanas después de completado el tratamiento. Se comparó la frecuencia de efectos adversos de medicamentos en trasplantados vs. no trasplantados. Los pacientes recibieron 9 esquemas de tratamiento diferentes. El genotipo más prevalente fue 1b (81.5%). La respuesta viral sostenida fue alcanzada por 186 pacientes (95.4%). El grupo no trasplantado tenía mayor frecuencia de cirrosis (52.6% vs. 12.5%, p = 0.0004). En los trasplantados, la carga viral pre-tratamiento era mayor (1 743 575 IQR = 1 038 062-4 252 719 vs. 345 769 IQR = 125 806-842 239; p = < 0.0001) igual que la ALT y la AST (82.5 IQR 43.5-115.5 vs. 37.0 IQR = 24.7-73.3; p = 0.0009 and 70 IQR = 41-140 vs. 37 IQR = 24-68; p = 0.004 respectivamente). El 28.7% refirió efectos adversos, siendo el más prevalente la astenia (5.6%). Nuestros resultados fueron comparables a los de estudios publicados en términos de terapia y biomarcadores pero nuestra cohorte presentó menos efectos adversos. Se requiere más investigación en la región.
Palabras clave: agentes antivirales, terapias combinadas, hepatitis C, hepatitis crónica, trasplante hepático, resultados de tratamiento
Recibido: 30-VIII-2018 Aceptado: 14-XII-2018
Postal address: Diego Rosselli, Departamento de Epidemiología y Bioestadística Clínica, Facultad de Medicina Pontificia Universidad Javeriana, Carrera 7 No. 40-62, Bogotá, Colombia
e-mail: diego.rosselli@gmail.com
The first attempt to ascertain the frequency of hepatitis C in Colombia dates back to 1992, when 4/497 (0.8%) health professionals were positive when voluntarily tested during a Congress of Internal Medicine held in Bogota1. In
1996, 10/430 serum samples from Tumaco, in the Pacific Coast, were positive both to an initial ELISA test and to a second confirmatory test2. In 1998, a prevalence study analyzed samples from 163 indigenous subjects from three different ethnic groups (inga, n = 15; kamsa, n = 54; wayuu, n = 94) and all were negative3. This apparently low hepatitis C prevalence in indigenous communities contrasted with that of hepatitis B, which is particularly high, as found by other studies in Amazonian communities of Brazil, Bolivia, Colombia, Peru, and Venezuela4. More recent studies, both from the Caribbean Coast5 and from Colombian Amazonian indigenous ethnic groups, have shown higher prevalence rates6.
In 1998, when the Pan American Health Organization warned about the risk of transmission of the virus through transfusions, Chile, Colombia, Costa Rica, and Venezu- ela had already made tests for the virus mandatory in all their blood banks7. The prevalence of the virus was then estimated at 0.45% in a sample of 41 575 blood donors in Santander8. In 2007, in a total of 6009 blood bank records from donations received in 2004-2005, 38 (0.6%) hepatitis C cases were detected by a third generation ELISA9. On the other hand, of 1840 Colombian women participating in a human papillomavirus study in 2015, 46 (2.5%) were positive for hepatitis C10.
A systematic review published in 200711 analyzed the prevalence of hepatitis C in intravenous drug abusers from 57 countries, and Bogota (with 2%), had the lowest figures in Latin America. On the other hand, Sepúlveda et al.12 found positive titers for hepatitis C in 16/71 (22.5%) intravenous drug abusers in a psychiatric hospital in Pereira, which contrasts with the finding of Bautista et al., who found none in a population of 259 illicit drug users in Bucaramanga (of which only 11 used intravenous drugs)13. In Armenia (Colombia), the prevalence was 22.3% in a sample of 265 intravenous drug users14.
With regards to genotype frequency, in 2002, in a sam- ple of 40 patients with hepatitis C from Medellin, Yepes et al. found that the predominant genotype and subtype were 1, and 1a, respectively, with an apparent relative increase of genotype 1b in the previous few years15, sug- gesting transfusions as the main transmission mechanism. In 2005, a study led by the Colombian National Institute of Health analyzed 500 patients who had received ten or more transfusions, from 4 blood banks in Bogota (n = 279) and Medellin (n = 221)16. The number of subjects sampled and the percentages that were positive, according to the 5 diagnoses included, were: cancer (n = 236, 3.4%), he- mophilia (n = 90, 32.2%), chronic hemodialysis (n = 82, 6.1%), acute bleeding (n = 78, 2%), and sickle cell anemia (n = 14, 7.1%). The study by Yepes et al. in the Caribbean Coast5 had found a history of transfusion in 44/55 affected individuals. Another study from a dialysis unit in Cali found reactivity in 29/999 patients (2.9%)17.
The distribution of hepatitis C subtypes in a sample of 185 positive sera from voluntary blood donors was: 1b (82.8%), 1a (5.7%), 2a (5.7%), 2b (2.8%), and 3a (2.8%)18. The most recent study of serotypes and genotypes, based on 1538 isolates of hepatitis C virus from 1527 patients, found genotype 1 in 88.6%, distributed as follows: subtype
1b 70%, subtype 1a 13.5 %, and not determined 5.1% of cases; genotype 2 was found in 5.4% of cases, 3 in 2%, and 4 in 4%; 0.8% had mixed genotypes19.
In Colombia, hepatitis C is the main indication for liver transplantation20. The natural history of hepatitis C changed radically with the development of direct-acting antiviral agents21-23. Their efficacy and safety have not been yet properly studied in Latin American population24. This study presents “real-world evidence” from hepatology centers in four Colombian cities.
Materials and methods
This retrospective multicenter study included all patients treated with direct-acting antiviral agents from five reference centers in hepatology, in four Colombian cities (Barranquilla, Bogota, Cartagena, and Medellin) during 2015-2017. Patients were not preselected according to any specific complaints and tests were done as part of their routine evaluation.
Adults with a diagnosis of hepatitis C were included in the study. The diagnosis of hepatitis C was defined as a positive test for anti-HCV antibodies confirmed by a positive HCV viral load. Additionally, patients should have initiated treatment with any of the available pharmacological schemes. Treatments were chosen by each treating hepatologist who was aware of the patient’s diagnosis, following national25 and international guidelines26-29. There were no excluded patients. Standard protocol approvals, registrations and patients’ consents were obtained.
A total of 195 patients were tested for HCV genotype, viral load, cirrhosis and fibrosis stage according to the fi- broscan scale. Serum biomarkers as bilirubin, ALT, AST, INR, hemoglobin, platelets and creatinine were measured. Child-Pugh and MELD scores were calculated for each pa- tient. All samples were taken under the routine examination and none of them was acquired during liver or renal crisis or under any acute illness. Samples were processed by the laboratory of the correspondent center from which the sample was obtained.
Patients were classified initially according to their treatment status into: patients who had not received any treatment, nonresponders, partial responders, patients with virologic relapse, patients with liver transplant. Patients were considered to be non-responders when the HCV RNA serum level remained detectable throughout the treatment and was formally defined as < 2 Log10 decline in HCV RNA between the baseline and week 1225. Patients were considered to have a virologic relapse when HCV RNA decreased and remained below the limit of detection during the treatment, but became detectable again after the discontinuation of the treatment25. Finally, patients were considered to be partial responders if the HCV RNA decreased ≥ 2 Log10 during the treatment but did not fulfill the responder requirements.
According to current practice, each patient had viral load quantified at the beginning and end of treatment, as well as at 12 weeks of completion, to ascertain sustained virologic response (SVR), which is considered the main outcome.
Additionally, all participants in the study were asked about adverse effects. These were classified in three categories: most common adverse effects, adverse effects that lead to the discontinuation of the treatment and serious adverse effects.
The following serious adverse effects were taken in account: liver decompensation, anemia, diarrhea, hepatotoxicity and kidney failure. Hepatotoxicity was defined as presenting hepatic encephalopathy, ascites, esophagus variceal bleeding and/or presenting a bilirubin level three times higher than the reference, or ALT/AST- ratio between concentration of aspartate transaminase (AST) and alanine transaminase (ALT) level five times higher than the reference.
Data were summarized and analyzed in two expert meetings. In the first one, the required sociodemographic and clinical information was agreed upon. In the second one, the entire data were analyzed, and missing, or inconsistent information was reviewed by the entire team. Information was summarized using commercially available
software Excel 2016 [Microsoft® Office 365®]. Statistical analyses were performed using commercially available software GraphPad Prism 6 [GraphPad Software Inc.]). Patients were considered separately according to their transplantation status and a comparison was made between transplanted and nontransplanted.
Furthermore, they were classified according the treatment they were receiving, and the virologic response as
well as the adverse effects described.
For comparison between transplanted and not transplanted patients, normality was assessed using Shapiro-
Wilk test. When the normality condition was satisfied, mean and standard deviation were reported. If the normality test was not satisfied, median and interquartile range were reported. For comparison between transplanted and not transplanted groups, the T-test with Welch correction was performed, and when proportions and nominal data were being compared, the Mann-Whitney test was used to compare two continuous distributions and/or non-parametric samples.
Following the indications of Resolution 8430 of 1993 from the Colombian Ministry of Health, which establishes national research ethics recommendations, this study was classified as “without risk”. Confidentiality was preserved throughout the study. The protocol was approved by the ethics committee and by each center’s institutional review board.
Results
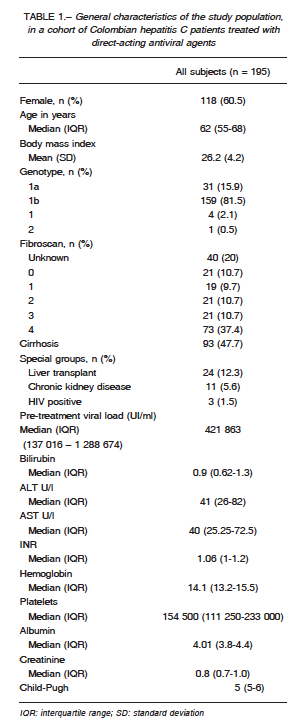
Information was collected from 195 subjects. Demographics are shown in Table 1. Almost two thirds were
women, and half of the patients were ≥ 62 years old.
They had been treated with 9 different regimens including paritaprevir/ritonavir-ombitasvir and dasabuvir
(ProD), daclatasvir/asunaprevir (DCV/ASV), ProD + rivirin (ProD + RBV), sofosbuvir/DCV + RBV (SOF/DCV + RBV), DCV/simprevir + RBV (DCV/SMV + RBV), SOF/DCV, SOF/ledipasvir + RBV (SOF/LDV + RBV) and SOF/RBV.
The most prevalent genotype was 1b (81.5%) and less than half of our cohort members presented cirrhosis.
Especial characteristics (liver transplant, chronic kidney disease, and HIV infection) were present in 19.5%. Baseline biomarkers of the entire cohort are described in Table 1.
Regarding previous treatment, 128 (65.6%) had not received any prior treatment, 42 (21.5%) were non-responders to previous treatment, 8 (4.1%) had received an unknown treatment, 13 (6.7%) had a virologic relapse, and 4 (2.1%) were partial responders; 24 patients (12.3%) had received a liver transplant.
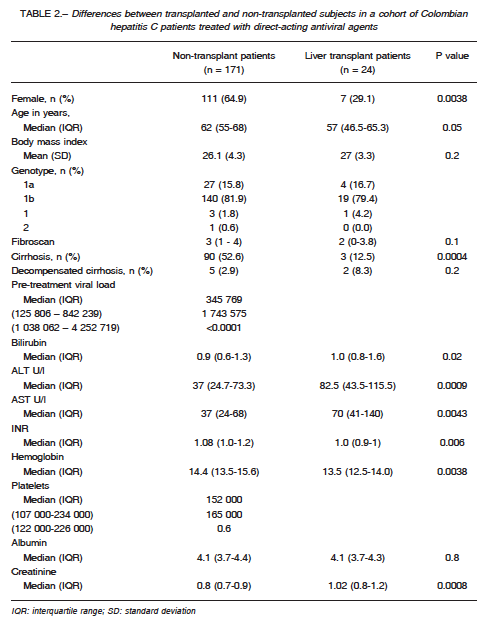
Comparison between patients with and without a liver transplant was made (Table 2). Concerning demographics, there were more females in the nontransplant group than in the transplant group and the most prevalent viral genotype in both groups was 1b.
Patients were comparable regarding body mass index (BMI), fibroscan score, and albumin levels. Members of the non-transplanted group were more likely to have cirrhosis than transplanted patients. Pre-treatment viral
load was higher in the transplanted group than in the non-transplanted group. Bilirubin was higher in the transplanted group than in the non-transplanted group, as well as ALT and AST. INR was higher in the nontransplanted
group, as well as hemoglobin and platelets.
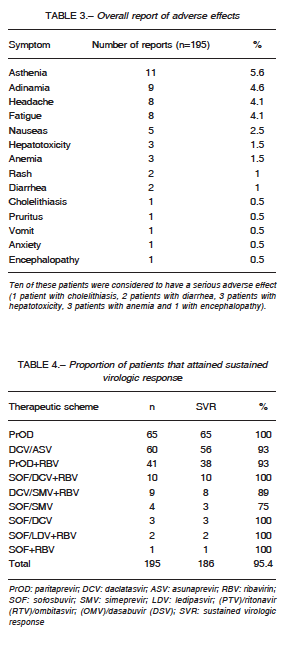
Creatinine was higher on the transplanted group. Regarding safety, 28.7% patients reported adverse effects, as summarized in Table 3. Of these, the most common were asthenia and adynamia. Ten were considered to have a serious adverse effect (encephalopa
thy 1, cholelithiasis 1, diarrhea 2, hepatotoxicity 3, and anemia 3). Six patients receiving PrOD + RBV (n = 3), DCV / ASV (n = 2) and DCV / SMV + RBV (n = 1) discontinued treatment because of adverse effects (cholelithiasis, diarrhea, and hepatotoxicity). However, 3 of them maintained a SVR despite discontinuing therapy.
Additionally, two of the transplant recipients (8.3%) stopped treatment because of hepatic decompensation;
both were receiving PrOD + RBV.
Most patients in our cohort were treated with ProD (33%). SVR was attained by 186 patients (95.4%). The
proportion attaining SVR according to each therapy is summarized in Table 4.
Discussion
To our knowledge this is the first Latin American study that provides real-world evidence for the efficacy of directacting
antiviral agents in the therapy of hepatitis C.
In the study population, as previously described in our country, the most frequent genotype is 1b (81.5%) followed
by 1a (15.9%), which suggests that the sample of this study is representative of Colombian cases, and that
having received a transfusion (prior to 1998) would be the main risk factor. The majority of the studied cases were
women, perhaps more prone to this particular risk factor.
Additional studies, however, are required in the target population to clarify the role of this and other risk factors
for hepatitis C virus transmission in Colombia.
A SVR was achieved by 95.4% (186/195) of our patients, which is comparable with the SVR described in the
literature for naive and treatment-experienced patients, which is usually 80-9026-29. At week 12 after starting treatment, 100% of the patients treated with ProD and SOF/ DCV+RBV presented SVR, which is comparable to figures
described for these therapies in other countries30. The association SOF/SMV presented the lowest response rate
in this cohort (75%), which is different from the virologic response found in other studies31. However, in our cohort
only 4 patients were being treated with this scheme and the small sample can be responsible for the difference.
Usually, “special patients” are considered difficult to treat as they have higher event rates and poor SVR rates32.
In this cohort, 122 cases (62.6%) were in this group, as they have presented with cirrhosis (n 93, 47.7%), with liver
transplant (24, 12.3%), with CKD (11, 5.6%), or co-infected with HIV (3, 1.5%). Despite this high percentage of special
patients, the entire cohort achieved an acceptable SVR.
Furthermore, a large proportion of patients (60, 30.8%) received medication schemes currently considered suboptimal
(in this case daclatasvir/asunaprevir) which was then the only option. The 93% SVR in these patients is similar to a large series of Korean cases treated with this
combination33. Other schemes with suboptimal response rates were daclatasvir/simeprevir + ribavirin, sofosbuvir/
simeprevir, and sofosbuvir/simeprevir.
Additionally, when comparing transplanted with nontransplanted patients, we found differences in terms of cirrhosis, pretreatment viral load, bilirubin, ALT and AST, INR, hemoglobin and platelets.
About viral load on the transplanted group, it has become clear in the literature that the recurrence of detection
of HCV RNA is nearly universal in transplanted patients34.
It has been described that viral load usually decreases immediately post-transplantation, but when patients are followed further in time, there is a significant increase reaching the pre-transplant viral load levels 48 hours
after the transplant and increasing up to 10 to 100 fold the pre-transplant viral load in the following month35,36, this
information is consistent with what is found in this cohort, with the transplanted patients viral load higher than the
non-transplanted ones.
Moreover, three patterns of recurrence have been de- scribed. In the first one, only transaminases are elevated as it refers to an acute hepatitis35. The second one is a chronic hepatitis which leads to cirrhosis in the 25% of reported presenting adverse effects (28.7%), contrary to what is described in the literature in which some cohorts reach even 90% of patients reporting adverse effects43, this could be due to under-reporting in this cohort due to different reasons as time given for the medical interview. Nevertheless, even when the percentage between the symptoms reported varies from study to study, the report- ed adverse effects are consistent with other reports44, 45. In this cohort, the most reported adverse effect was asthenia, followed by adynamia, headache, fatigue and nausea.
A limitation of this study is the quality of information collected, a common problem in real-world evidence stud- ies. All participant researchers used common and well stablished criteria for diagnosis and follow-up, which gives credibility to our data on efficacy. Safety, however, seems to be a serious problem, since information on adverse events was not recorded properly in the clinical records, except for major adverse events, or those that required interruption of treatment.
Further research in Colombia should point towards the characterization of the safety of the regimes and correla- tion between precise regimes, specific adverse effect and the premature discontinuation of each regime, in order to provide better quality information.
Conflict of interests: This study was supported by Ab- bvie Colombia
References
1. Botero R, Urdaneta F, Sirutis D. Seroprevalencia de mar- cadores de hepatitis B y C en trabajadores del área de salud. Acta Méd Colomb 1994; 19: 62-75.
2. Robinson JW, Rosas M, Guzman F, Patarroyo M, Moreno A. Comparison of prevalence of anti-hepatitis C virus antibodies in differing South American populations. J Med Virol 1996; 50: 188-92.
3. Tanaka Y, Mizokami M, Orito E, et al. GB virus C/hepatitis G virus infection among Colombian native Indians. Am J Trop Med Hyg 1998; 59: 462-7.
4. Echevarría JM, León P. Epidemiology of viruses causing chronic hepatitis among populations from the Amazon Basin and related ecosystems. Cad Saúde Publica 2003; 19: 1583-91.
5. Yepes IJ, Lince B, Caez C, De Vuono G. Factores de riesgo para la infección por el virus de la hepatitis C en la Costa Caribe colombiana: un estudio de casos y controles. Biomédica 2016; 36: 564-71.
6. Alvarado-Mora MV, Fernandez MF, Gomes-Gouvêa MS, de Azevedo Neto RS, Carrilho FJ, Pinho JR. Hepatitis B (HBV), hepatitis C (HCV) and hepatitis delta (HDV) viruses in the Colombian population – How is the epide- miological situation? PLoS One 2011; 6: e1888.
7. Schmunis G, Zicker F, Pinheiro F, Brandling-Bennett D. Risk for transfusion-transmitted infectious diseases in
Central and South America. Emerg Infect Dis 1998; 4: 5-11.
8. Gómez L, Peñuela O, Higuera F. Prevalence of antibod- ies against transfusion-transmissible infections (TTI) in blood donors from the Colombian eastern region. Clin Lab 2014; 60: 869-71.
9. Farfan Y, Garzon M, Rey M, Molano J, Lizarazo J, Maru- landa J. Prevalence of hepatitis C by RT-PCR in donors of the blood bank. Rev Col Gastroenterol 2007; 22: 308-
12.
10. Quesada P, Whitby D, Benavente Y, et al. Hepatitis C virus seroprevalence in the general female population from 8 countries. J Clin Virol 2015; 68: 89-93.
11. Aceijas C, Rhodes T. Global estimates of prevalence of HCV infection among injecting drug users. Int J Drug
Policy 2007; 18: 352-8.
12. Sepúlveda-Arias J, Isaza C, Vélez J. Hepatitis B and C prevalence among heroin addicts in methadone maintenance treatment (MMT) and not in MMT in Pereira, Colombia. J Infect Dev Ctries 2014; 8: 1228-30.
13. Bautista-Amorocho H, Jaimes Moreno B, Hincapié López M. Ausencia de infección por virus de la hepatitis C en usuarios de drogas ilícitas en la ciudad de Bucaramanga, Colombia. Rev Colomb Gastroenterol 2011; 26: 15-20.
14. Berbesi-Fernández D, Segura-Cardona Á, Montoya-Vélez L, Castaño-Perez G. Hepatitis C and HIV in injecting drug users in Armenia, Colombia. Adicciones 2015; 27: 246-52.
15. Yepes A, Alvarez C, Restrepo J, Correa G, Zapata J, Arango AE. Genotipos virales en pacientes con infec- ción por el virus de la hepatitis C (VHC) en Medellín. Gastroenterol Hepatol 2002; 25: 334-5.
16. Beltrán M, Navas M, De la Hoz F, et al. Hepatitis C virus seroprevalence in multi-transfused patients in Colombia. J Clin Virol 2005; 34: S33-8.
17. Ramírez R, Fernández J, Guevara J, et al. Prevalencia de anticuerpos contra el virus de hepatitis C en unidades de diálisis de Cali-Colombia. Rev Col Gastroenterol 2010; 25: 14-8.
18. Mora M, Romano C, Gomes-Gouvêa M, Gutiérrez M, Carrilho F, Pinho J. Molecular characterization, distribu- tion, and dynamics of hepatitis C virus genotypes in blood donors in Colombia. J Med Virol 2010; 82: 1889-98.
19. Santos Ó, Gómez A, Vizcaíno V, Casas MC, Ramirez MP, Olaya P. Genotipos circulantes del virus de la hepatitis
C en Colombia. Biomédica 2017; 37: 22-7.
20. Bhamidimarri K, Satapathy S, Martin P. Hepatitis C virus and liver transplantation. Gastroenterol Hepatol (N Y)
2017; 13: 214-20.
21. Morio K, Imamura M, Kawakami Y, et al. Real-world ef- ficacy and safety of daclatasvir and asunaprevir therapy for hepatitis C virus-infected cirrhosis patients. J Gastro- enterol Hepatol 2017; 32: 645-50.
22. Welzel T, Petersen J, Herzer K, et al. Daclatasvir plus so- fosbuvir, with or without ribavirin, achieved high sustained virological response rates in patients with HCV infection and advanced liver disease in a real-world cohort. Gut 2016; 65: 1861-70.
23. Pol S, Corouge M, Vallet-Pichard A. Daclatasvir-sofos- buvir combination therapy with or without ribavirin for hepatitis C virus infection: from the clinical trials to real life. Hepat Med 2016; 8: 21-6.
24. Hernández-Vásquez A, Rosselli D. A bibliometric analysis of the global research on sofosbuvir. F1000Res 2017; 6:
1536.
25. Dieterich T, Rizzetto M, Manns MP. Management of Chronic hepatitis c patients who have relapsed or not responded to pegylated interferon alfa plus ribavirin D. J Viral Hepat 2009; 16: 833-43.
26. Jacobson I, Dore G, Foster G, et al. Simeprevir with pe- gylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infec- tion (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet 384: 403-13.
27. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previ- ously untreated chronic hepatitis C infection. N Engl J Med 2013; 368: 1878-87.
28. Afdhal N, Zezem S, Kwo P, et al. Ledipasvir and sofos buvir for untreated HCV genotype 1 infection. N Engl J
Med 2014; 370: 1889-98.
29. Smith-Palmer J, Cerri K, Valentine W. Achieving sus- tained virologic response in hepatitis C : a systematic review of the clinical, economic, and quality of life ben- efits. BMC Infect Dis 2015; 15:1-19.
30. Weil C, Chodick G, Mehta D, et al. Sustained virological response to ombitasvir / paritaprevir / ritonavir and das- abuvir treatment for hepatitis C: Real-world data from a large healthcare provider. J Viral Hepat 2018; 25: 144-51.
31. Sclair SN, Hernandez MD, Vance E, et al. Sofosbuvir and simeprevir combination therapy for HCV genotype
1 infection: Results of a single-center VA experience. Gastroenterol Hepatol (N Y) 2016; 12: 490-7.
32. Mendez-Sanchez N, Parana R, Cheinquer H, et al. Latin American Association for the Study of the Liver recom- mendations on treatment of hepatitis C. Ann Hepatol 2014; 13: s4-66.
33. Cho BW, Kim SB, Song IH, et al. Efficacy and safety of daclatasvir plus asunaprevir for Korean patients with HCV genotype Ib infection : a retrospective multi-institutional study. Clin Mol Hepatol 2017; 23: 51-6.
34. Wright TL, Hsu HH, Ferrell L, et al. Recurrent and ac- quired hepatitis C viral infection in liver transplant recipi- ents. Gastroenterology 1992; 103: 317-22.
35. Tsoulfas G, Goulis I, Giakoustidis D, et al. Hepatitis C and liver transplantation. Hippokratia 2009; 13: 211-5.
36. Seekumar R, Gonzalez-Koch A, Maor-Kendler Y, et al. Early identification of recipients with progressive histo- logic recurrence of hepatitis C After liver transplantation. Hepatology 2000; 32: 1125-30.
37. Wiesner RH, Sorrell M, Villamil F, et al. Report of the First International Liver Transplantation Society Expert Panel Consensus Conference on Liver Transplantation and Hepatitis C. Liver Transplantation 2003; 9: 1-9.
38. Maheshwari A, Mishra R, Thuluvath PJ. Post-liver-trans- plant anemia: Etiology and management. Liver Transplant 2004; 10: 165-73.
39. Ampuero J, Romero-Gomez M. Pharmacogenomics of ribavirin-induced anemia in hepatitis C. Pharmacogenom- ics 2016; 17: 1587-94.
40. Olariu M, Olariu C, Olteanu D. Thrombocytopenia in Chron- ic Hepatitis C. J Gastrointestin Liver Dis 2010; 19: 381-5.
41. Stanca CM, Fiel MI, Aledort L, Cohen E, Martin JR, Schiano TD. Factors associated with persi bstent throm- bocytopenia after liver transplantation. Transplant Proc 2010; 42: 1769-73.
42. Sutedja DS, Wai C, Teoh K, et al. Persistent thrombocy- topenia in liver transplant patients. Transplant Proc 2004; 36: 2331-3.
43. Medeiros T, Salviato CM, do Rosário NF. Adverse ef- fects of direct acting antiviral-based regimens in chronic hepatitis C patients : a Brazilian experience. Int J Clin Pharm 2017; 39: 1304-11.
44. Gane EJ, Kowdley K V, Pound D, et al. Efficacy of sofos- buvir, velpatasvir, and GS-9857 in patients with hepatitis C virus genotype 2, 3, 4, or 6 infections in an open-label, phase 2 trial. Gastroenterology 2016; 151: 902-9.
45. Werner CR, Schwarz JM, Egetemeyr DP, et al. Second- generation direct-acting-antiviral hepatitis C virus treat- ment: Efficacy, safety, and predictors of SVR12. World J Gastroenterol 2016; 22: 8050-9.
– – – –
[…] La civilización, bajo su aspecto moral, es un conjunto de cualidades ar- tificialmente desarrolladas, proviniendo de aquí la diferencia entre el individuo civilizado y el salvaje. Éste depende del medio en que ha nacido; el otro es su colaborador inteligente.
Leopoldo Lugones (1874-1938)
El Imperio Jesuítico (1904). Buenos Aires: Hyspamérica, 1985. Epílogo, p 251
