*en inglés
NATALIA A. CASANOVA1, MARÍA FERNANDA SIMONIELLO2,
¹ Universidad de Buenos Aires, Facultad de Farmacia y Bioquímica, Instituto de Fisiopatología y Bioquímica Clínica (INFIBIOC), Departamento de Bioquímica Clínica, Citogenética Humana y Genética Toxicológica (CIGETOX)
² Cátedra de Toxicología, Farmacología y Bioquímica Legal, Facultad de Bioquímica y Ciencias Biológicas, Universidad Nacional del Litoral, Santa Fe, Argentina
Resumen Efecto modulador del berro sobre el estrés oxidativo inducido por ciclofosfamida en ratones. El berro (Nasturtium officinale, crucíferas; W. Aiton) es una hortaliza ampliamente consumida en nuestro país, con valor nutricional y propiedades potencialmente quimiopreventivas. En trabajos previos demostramos que el jugo de berro tiene efecto protector in vivo contra el daño del ADN inducido por ciclofosfamida en tejidos del ratón. En el presente trabajo evaluamos, también in vivo, los efectos del jugo sobre el estrés oxidativo en diferentes tejidos del ratón. Los siguientes biomarcadores fueron investigados: actividad de superóxido dismutasa, actividad de catalasa, peroxidación lipídica y balance de glutatión. Los animales fueron tratados con diferentes dosis de jugo (0.5 y 1 g/kg de peso corporal) por alimentación forzada durante 15 días consecutivos antes de la inyección intraperitoneal con ciclofosfamida (100 mg/kg). La ingesta de berro antes de la administración de ciclofosfamida mejoró la actividad de superóxido dismutasa en los eritrocitos sin efecto sobre la actividad de la catalasa. En médula ósea e hígado, el jugo de berro contrarrestó el efecto deletéreo de la ciclofosfamida. En todas las matrices, el balance de glutatión fue mayor y la oxidación de lípidos menor que los valores encontrados en los grupos control. Nuestros resultados demuestran que el berro es un componente de la dieta con propiedades prometedoras como promotor de la salud o como agente protector contra el daño oxidativo.
Palabras clave: defensa antioxidante, quimioprevención, estrés oxidativo, berro
Abstract
Watercress (Nasturtium officinale, Cruciferae; W. Aiton) is a vegetable widely consumed in our country, with nutritional and potentially chemopreventive properties. Previous reports from our laboratory demonstrated the protective effect of watercress juice against DNA damage induced by cyclophosphamide in vivo. In this study, we evaluated the in vivo effect of cress plant on the oxidative stress in mice. Animals were treated by gavage with different doses of watercress juice (0.5 and 1g/kg body weight) for 15 consecutive days before intraperitoneal injection of cyclophosphamide (100 mg/kg body weight). After 24 h, mice were killed by cervical dislocation. The effect of watercress was investigated by assessing the following oxidative stress biomarkers: catalase activity, superoxide dismutase activity, lipid peroxidation, and glutathione balance. Intake of watercress prior to cyclophosphamide administration enhanced superoxide dismutase activity in erythrocytes with no effect on catalase activity. In bone marrow and liver tissues, watercress juice counteracted the effect of cyclophosphamide. Glutathione balance rose by watercress supplementation and lipid oxidation diminished in all matrixes when compared to the respective control groups. Our results support the role of watercress as a diet component with promising properties to be used as health promoter or protective agent against oxidative damage.
Key words: antioxidants, chemoprevention, oxidative stress, watercress
Received: 12-IX-2016 Accepted: 23-III-2017
Postal address: Dra. Marta A. Carballo, INFIBIOC, Facultad de Farmacia y Bioquímica, UBA, Junín 956, 1113 Buenos Aires, Argentina
e-mail: macarballo@ffyb.uba.ar
Watercress (Nasturtium officinale, Cruciferae; W. Aiton) is a widely consumed cruciferous vegetable with nutritional and potentially chemopreventive properties. More specifically, this vegetable reduces oxidative DNA damage and modulates antioxidant enzymes in human cells, according to the results obtained in diverse in vitro and in vivo models1-4. This ability has been attributed to isothiocyanates, the main phytochemical compounds present in cruciferae. However, paradoxical effects of isothiocyanates have been reported in relation with oxidative stress and the metabolites involved5.
Cyclophosphamide (CP), a bifunctional alkylating agent, is widely used in the treatment of cancer and other conditions such as rheumatoid arthritis, multiple sclerosis, systemic lupus erythematosus, among others. It is classified as a known human carcinogen based on the extensive evidence found not only in experimental animals but also in humans6.
CP is classified and used as a model for the study of indirect mutagens due to the fact that the molecule needs to be metabolized to generate its toxic effects. The drug is converted into products with cytotoxic and alkylating activity via the P-450-dependent mixed-function oxidase system. CP is transformed into 4-hydroxicyclophosphamide and aldophosphamide, which in turn generate phosphoramide mustard and acrolein, among other metabolites7. These metabolites interact with DNA and form adducts and cross-links that interfere with DNA replication and repair mechanisms8, 9. Likewise, CP induces oxidative stress and cell death10. Thus, it is important to highlight that the CP alkylating activity is responsible for its therapeutic activity. However, metabolites like acrolein induce oxidative stress which leads to DNA damage of normal cells and toxicity to various target organs11.
Chemoprevention is a substantial strategy to lessen or avoid damage of normal cells that inevitably occur by exposure to genotoxic and oxidative agents present in air pollutants, chemotherapy or diet. In previous studies, our group demonstrated that watercress juice seems to have a protective effect on peripheral blood lymphocytes against hydrogen peroxide-induced DNA damage in vitro12. Furthermore, we found that watercress-supplemented diet exhibited protective activity against the DNA damaging effect induced by the indirectly acting alkylating agent CP, as measured in vivo by comet and micronucleus assays13. Encouraged by these results, we designed the present study to investigate the effects of watercress on the oxidative damage induced in vivo by CP in Swiss albino mice. The experimental end points included catalase activity, superoxide dismutase activity, levels of thiobarbituric acid reactive substances as expression of lipid peroxidation, and ratio of reduced glutathione to oxidized glutathione.
Materials and methods
All chemicals were purchased from Sigma (St. Louis, MO, USA) unless otherwise indicated.
Watercress was purchased from an organic market garden located in Luján, Buenos Aires, Argentina, and voucher specimens were kept at the Museum of Pharmacobotany Juan A. Domínguez, School of Pharmacy and Biochemistry, University of Buenos Aires. The juice of watercress leaves was prepared on ice and protected from light using a commercial processor (Ultracomb). The homogenate was centrifuged at 10 000 g for 20 minutes at 4 °C. The supernatants were clarified by filtration using 0.45 μm pore membranes (Millipore) and sterilized by microfiltration using 0.22 μm pore membranes (Millipore).
Seven to eight weeks old Swiss mice (25-30 g) supplied by the Animal House of the School of Pharmacy and Biochemistry, University of Buenos Aires, were housed in plastic cages at 20-25 °C under 12 h light-dark cycles. The animals were acclimated for a week before the study onset and received food and water ad libitum through the entire experimental period. In all experiments, animal use and care were in accordance with ethical laws on animal manipulation.
A previous study from our lab showed that watercress protected against the genotoxic effects of CP, considering Nasturtium officinale human daily consumption (75-125 g)12. The dose of CP (100 mg/kg b.w.) was selected on the basis of a pilot study and reports from other groups16, 17. The doses of watercress used in this experimental design were 0.5 and 1g/kg b.w.18. The mice (n = 4 per group) were given either saline solution (groups I and II) or watercress juice (groups III and IV) by gavage for 15 consecutive days prior to the intraperitoneal administration of saline solution (I) or CP (II, III and IV). All animals were killed by cervical dislocation 24 h after CP administration.
Blood samples were collected by cardiac puncture with a heparinized syringe immediately after death. Red blood cells were separated by centrifugation at 1000 g for 15 min at 4 °C followed by removal of plasma and buffy coat. The erythrocytes were washed three times with saline solution, and stored at -20 °C until use. Hemoglobin concentration was determined with a Sysmex XS 1000i automatic hematology analyzer at 555 nm.
Bone marrow cells from both femurs of each animal were flushed in the form of fine suspension into a centrifuge tube containing RPMI 1640 medium (Gibco BRL). Cell suspensions were centrifuged at 1000 g for 10 min and pellets were lysed by two freezing-thawing cycles followed by homogenization by repeated passage through a 21G needle and centrifugation at 1000 g. Protein concentration was assayed by a colorimetric method19.
Livers were properly cleaned, freed from connective tissue, and dried on filter paper. With livers preserved at -80 °C, homogenates were prepared according to Sabatini et al.20. Briefly, livers were homogenized with 0.134 M KCl (1:5 w/v) containing 0.5 mM phenylmethylsulfonyl fluoride and 0.2 mM benzamidine (protease inhibitors) to study oxidative stress parameters. Homogenates were centrifuged at 11 000 g for 20 min. Biochemical determinations were carried out in supernatants of total homogenates and total soluble protein content was determined in each homogenate19.
For enzyme assays, erythrocytes were hemolyzed by adding ice-cold water at a 1/100 dilution; bone marrow suspensions and liver homogenates were diluted 1/10. Catalase activity was evaluated according to Aebi21. Briefly, a mixture composed of 50 mM phosphate buffer pH 7.0, 53 mM hydrogen peroxide and samples were sequentially measured at 240 nm with a Hitachi 3000 spectrophotometer for 1 min at 25 °C to assess the reduction rate of hydrogen peroxide. One unit of catalase was defined as the amount of enzyme that decomposed 1 mole of hydrogen peroxide per min. The specific activity was expressed as kU/g Hb in erythrocytes and as U/mg protein in tissues. Superoxide dismutase activity was measured using a commercial kit (SOD determination kit, Sigma Aldrich) according to the manufacturer’s instructions and expressed as percentage inhibition.
Lipid peroxidation was determined as malondialdehyde according to Ohkawa et al.22. Each sample was added to a 8% SDS solution, followed by addition of 20% acetic acid and 0.8% thiobarbituric acid. Distilled water was added to a final volume of 1 ml. Then, samples were incubated in a 100 °C water bath for 1h. The mixture was cooled and centrifuged at 10 000 g for 15 min. The supernatant was used to determine the absorbance at 532 nm with a Hitachi 3000 spectrophotometer.
Lipid peroxidation was measured as concentration of thiobarbituric acid reactive substances, calculated using the extinction coefficient 1.56 x 105/M x cm and expressed as nmol/g Hb or nmol/g protein.
The ratio of reduced glutathione to oxidized glutathione was assayed using the method described earlier23. Briefly, reduced glutathione was measured by a modification of Ellman´s procedure24 with 1.5 mM dithiobisnitrobenzoate in 0.25 M phosphate buffer (pH 8.0) and 0.1 ml of supernatant (total volume: 1.1ml). The mixture was incubated for 2 minutes at 25 °C and the absorbance was measured at 412 nm using a Hitachi 3000 spectrophotometer.
In order to estimate the total content of glutathione (GSSG + GSH) the oxidized form was reduced with glutathione reductase (EC 1.6.4.2) and 0.21 mM NADPH in 143 mM phosphate buffer plus EDTA (pH 7.5). After 30 minutes of incubation at 25 °C, the reaction was stopped with trichloroacetic acid and the supernatant was used to determine the absorbance as described above.
Results were shown as mean ± SD for all the endpoints. Differences between controls and treatments were analyzed by one-way analysis of variance and the post-hoc Student-Newman-Keuls test. A value of p < 0.05 was considered as statistically significant (Sigma Stat 9.0 software).
Results
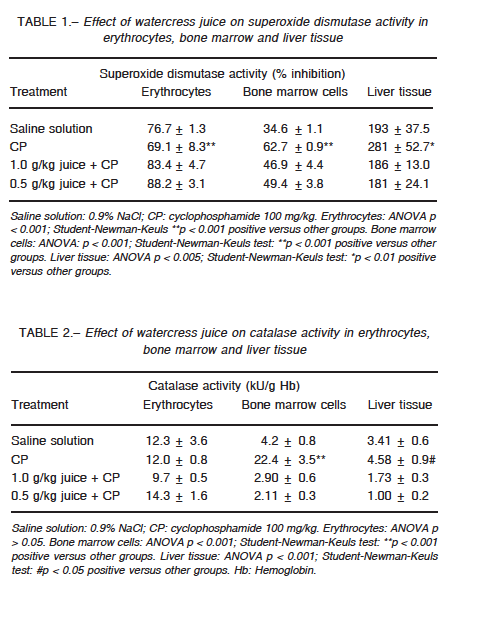
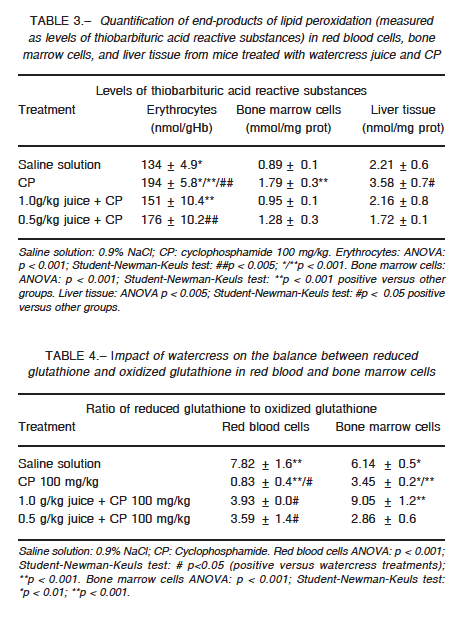
Erythrocyte catalase did not show any significant change in its activity for any of the groups, whereas the activity of superoxide dismutase was altered in the groups treated with CP (p < 0.001) and combined treatment (p < 0.001) when compared with the control group (Tables 1 and 2). In bone marrow, CP administration increased the activity of both enzymes as compared to the control group (p < 0.001), while cress juice attenuated these effects (p < 0.001) (Tables 1 and 2). A similar pattern was observed for the activity of both antioxidant enzymes in liver tissue (catalase: p < 0.05; superoxide dismutase: p < 0.01; Tables 1 and 2). CP led to a considerable increase in the levels of thiobarbituric acid reactive substances (p < 0.001); significant restoration was observed in animals that received watercress supplementation as compared to animals that received CP alone (1 g/kg: p < 0.001; 0.5 g/kg in erythrocytes (p < 0.005), bone marrow (p < 0.001), and liver tissue (p < 0.05) (Table 3). Moreover, CP altered the ratio of reduced glutathione to oxidized glutathione and watercress supplementation restored, at least in part, the balance in blood cells and bone marrow (p < 0.05; p < 0.001 respectively) (Table 4).
Discussion
Natural products are remarkable sources of biologically active molecules that could modulate oxidative stress26. Cruciferous vegetables such as broccoli, cabbage, cauliflower, Brussels sprouts and watercress, among others, have been extensively studied in relation to their ability to ameliorate or prevent the injuries caused by oxidation in living cells. Particularly, watercress is rich in vitamin C, vitamin A and α-tocopherol. It contains high concentrations of glucosinolates as well as carotenoids, polyphenols and chlorophyll14. The analysis of watercress leaves indicated the presence of gluconasturtiin or phenethylglucosinolate, methylsulfinylalkyl and indole glucosinolates. Furthermore, very low levels of glucobarbarin were found while neither sinalbin nor gluconapin was detected. The total glucosinolate content was 15 ± 4 μmol glucosinolates/g dry weight with no differences in relation to harvest time25.
In the present work, we evaluated the modulator effect of watercress juice intake on the oxidative stress induced by CP in erythrocytes, liver tissue and bone marrow cells of Swiss mice. CP induced different responses in cells with respect to basal enzymatic activities. In erythrocytes, superoxide dismutase activity decreased without any effect on catalase activity, while both enzymes increased their activity in bone marrow and liver tissue. As for the other two parameters studied, the same pattern was observed: increased levels of lipid peroxidation and decreased ratio of reduced glutathione to oxidized glutathione.
Changes in erythrocyte superoxide dismutase activity induced by CP may be due to alterations in the synthesis or structure of the enzyme and/or by the effect of the hydrogen peroxide produced by the drug and not degraded by catalase. This impaired defense would be linked, at least in part, to high levels of lipid peroxidation. Likewise, the altered ratio of reduced glutathione to oxidized glutathione could be caused by an increase in the oxidized form or conjugation of CP or its metabolites with glutathione in its reduced form. In bone marrow and liver tissues, changes in the activity of the enzymes could be explained as a compensatory mechanism to injury, where the oxidative atmosphere generated by the drug induces the expression and/or activity of antioxidant enzymes. However, the response did not appear to be sufficient to deal with the stress, which is reflected by the increase observed in lipid peroxidation. On the other hand, CP reacts indirectly with the reduced form of glutathione forming an adduct, which induces the formation of oxygen radicals27. The enzymes xanthine oxidase and aldehyde dehydrogenase interact with the adduct and produce superoxide and hydroxyl, which exacerbate the oxidative trend28.
The ability of watercress to modulate the oxidative effect of CP in terms of the enzymatic activity exhibits a hormetic pattern. In erythrocytes, the greatest superoxide dismutase response was obtained with 0.5 g/kg while the highest dose decreased its activity with no modification in catalase performance. In bone marrow and liver tissue, the juice intake prevented the compensatory response observed in the CP-treated group for both enzymes.
Watercress restored the content of the reduced glutathione in erythrocytes exposed to CP. On the other hand, a biphasic response was observed in bone marrow, increasing this parameter at 0.5 g/kg dose and decreasing it at 1 g/kg dose. The lower dose was not enough to counter the impact of the drug while higher doses achieved the desired effect. In addition, in all cases watercress supplementation attenuated the peroxidation induced by the drug. Our results are consistent with those obtained by others29 where the administration of watercress to hypercholesterolemic rats was shown to induced a decrease in malondialdehyde levels, an index of lipid peroxidation attenuation.
It is well known that the main glucosinolate in watercress is phenethylglucosinolate, commonly named as gluconasturtiin. It has been reported that this compound can act as a genotoxic agent to Saccharomyces cerevisiae and in mice inhibits and/or enhances phase I and phase II enzymes activities, respectively31. However, there are other phytochemicals in this vegetable such as methylsulfinylalkyl and methylthioalkyl glucosinolates which have demonstrated health-promoting effects32.
Several reports describe the association between cruciferous consumption and health. The International Agency for Research on Cancer (IARC) published a handbook of cancer prevention which focuses exclusively on crucifers and their metabolites demonstrating the importance of this plant family in health promotion31. The National Cancer Institute also recommends the intake of these vegetables because of the beneficial properties of their phytochemicals33.
It is noteworthy that the doses used in this experimental design are within the limits allowed by the protocol guidelines for in vivo tests18. These doses are much lower than the concentrations tested in our previous in vitro experimental research, which represent the average plant intake for an average adult human12. The effect against oxidative damage observed in vivo at such low doses highlights the great capacity of the plant as a chemopreventive agent. Our evidence supports the consumption of watercress as a diet component with promising properties, to be used as a health promoter or protective agent. It is important to emphasize that the effects always depend on the vegetable species, its origin and growing conditions, its composition, doses employed, tissue analyzed and exposure time to the agent.
Acknowledgements: The authors thank Graciela Spivak for insightful discussions and comments on the manuscript. The authors are thankful to Dr. Niels Agerbirk for watercress analysis and to the staff of the Animal House, School of Pharmacy and Biochemistry of the University of Buenos Aires for providing and keeping the animals used in these experiments.
This study was funded by grant 20020130100326BA 2014-2017 of the University of Buenos Aires.
Conflict of interests: None to declare
References
1. Boyd L, McCann M, Hashim Y, Bennett RN, Gill CI, Rowland IR. Assessment of the anti-genotoxic, anti-proliferative and anti-metastatic potential of crude watercress extract in human colon cancer cells. Nutr Cancer 2006; 55: 232-41.
2. Gill CI, Haldar S, Boyd LA, et al. Watercress supplementation in diet reduces lymphocyte DNA damage and alters blood antioxidant status in healthy adults. Am J Clin Nutr 2007; 85: 504-10.
3. Hofmann T, Kuhnert A, Schubert A, et al. Modulation of detoxification enzymes by watercress: in vitro and in vivo investigations in human peripheral blood cells. Eur J Nutr 2009; 48: 483-91.
4. Zhu CY, Loft S. Effects of Brussels sprouts extracts on hydrogen peroxide-induced DNA strand breaks in human lymphocytes. Food Chem Toxicol 2001; 39: 1191-7.
5. Zhang Y, Li J, Tang L. Cancer-preventive isothiocyanates: dichotomous modulators of oxidative stress. Free Radic Biol Med 2005; 38: 70-7.
6. IARC Working Group on the evaluation of carcinogenic risks to humans. Pharmaceuticals, Volume 100A. A review of human carcinogens. International Agency for Research on Cancer ed. Lyon, France 2012, p 63-90. In: http://monographs.iarc.fr/ENG/Monographs/vol100A/mono100A.pdf; accessed June 2013.
7. Florez J, Armijo JA, Mediavilla AM. Metabolismo de los fármacos. Farmacología humana, 6th ed. España: Elsevier Health Sciences Spain, 2013, p 73-86.
8. Gilani SH, Chatzinoff M. Embryopathic effects of cyclophosphamide. Environ Res 1983; 31: 296-301.
9. Enns GM, Roeder E, Chan RT, Ali-Khan Catts Z, Cox VA, Golabi M. Apparent cyclophosphamide (cytoxan) embryopathy: a distinct phenotype? Am J Med Genet 1999; 86: 237-41.
10. Rehman MU, Tahir M, Ali F, et al. Cyclophosphamide-induced nephrotoxicity, genotoxicity, and damage in kidney genomic DNA of Swiss albino mice: the protective effect of Ellagic acid. Mol Cell Biochem 2012; 365: 119-27.
11. Korkmaz A, Topal T, Oter S. Pathophysiological aspects of cyclophosphamide and ifosfamide induced hemorrhagic cystitis; implication of reactive oxygen and nitrogen species as well as PARP activation. Cell Biol Toxicol 2007; 23: 303-12.
12. Casanova NA, Carballo MA. Antigenotoxic activity of watercress extract in an in vitro mammalian system using comet assay. Phytother Res 2011; 25: 1743-6.
13. Casanova NA, Ariagno J, López Nigro M, et al. In vivo antigenotoxic activity of watercress juice (Nasturtium officinale L.) against induced DNA damage. J Appl Toxicol 2013; 33: 880-5.
14. Casanova NA, López Nigro M, Wagner M, Carballo M. Effect of watercress on induced DNA damage, DNA repair and p-glycoprotein activity in human lymphocytes. J Basic Appl Genet 2014; 25: 53-60.
15. Agerbirk N, Olsen CE. Isoferuloyl derivatives of five seed glucosinolates in the crucifer genus Barbarea. Phytochemistry 2011; 72: 610-23.
16. Zhang QH, Wu CF, Duan L, Yang JY. Protective effects of ginsenoside Rg(3) against cyclophosphamide-induced DNA damage and cell apoptosis in mice. Arch Toxicol 2008; 82: 117-23.
17. Tripathi DN, Jena GB. Intervention of astaxanthin against cyclophosphamide-induced oxidative stress and DNA damage: A study in mice. Chem Biol Interact 2009; 180: 398-406.
18. Organization for Economic Co-operation and Development. (1997). OECD Guideline for the testing of chemicals Nº 474. Mammalian Erythrocyte Micronucleus Test. In: http://www.oecd.org/chemicalsafety/risk-assessment/1948442.pdf; accessed July 2010.
19. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248-54.
20. Sabatini S, Rocchetta I, Nahabedian D, et al. Oxidative stress and histological alterations produced by dietary copper in the fresh water bivalve Diplodon chilensis. Comp Biochem Physiol C Toxicol Pharmacol 2011; 154: 391-8.
21. Aebi H. Catalase in vitro. Methods Enzymol 1984; 105: 121-6.
22. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979; 95: 351-8.
23. Venturino A, Anguiano OL, Gauna L, Cocca C, Bergoc RM, Pechen de D’Angelo AM. Thiols and polyamines in the potentiation of malathion toxicity in larval stages of the toad Bufo arenarum. Comp Biochem Physiol C Toxicol Pharmacol 2001; 130: 191-8.
24. Ellman GL. Tissue sulphydryl groups. Arch Biochem Biophys 1959; 82: 70-7.
25. Agerbirk N, Olsen C, Cipollini D, Ørgaard M, Linde-Laursen I, Chew FS. Specific glucosinolate analysis reveals variable levels of epimeric glucobarbarins, dietary precursors of 5-phenyloxazolidine-2-thiones, in watercress types with contrasting chromosome numbers. J Agric Food Chem 2014; 62: 9586-96.
26. Kapiszewska M, So£tys E, Visioli F, Cierniak A, Zajac G. The protective ability of the Mediterranean plant extracts against the oxidative DNA damage. The role of the radical oxygen species and the polyphenol content. J Physiol Pharmacol 2005; 56 Suppl 1: 183-97.
27. Perry CS, Liu X, Lund LG, Whitman CP, Kehrer JP. Differential toxicities of cyclophosphamide and its glutathione metabolites to A549 cells. Toxicol in vitro 1995; 9: 21-6.
28. Adams J, Klaidman L. Acrolein-induced oxygen radical formation. Free Radic Biol Med 1993; 15: 187-93.
29. Yazdanparast R, Bahramikia S, Ardestani A. Nasturtium officinale reduces oxidative stress and enhances antioxidant capacity in hypercholesterolaemic rats. Chem Biol Interact 2008; 172: 176-84.
30. Canistro D, Croce CD, Iori R, et al. Genetic and metabolic effects of gluconasturtiin, a glucosinolate derived from cruciferae. Mutat Res 2004; 545: 23-35.
31. Rose P, Faulkner K, Williamson G, Mithen R. 7-Methylsulfinylheptyl and 8-methylsulfinyloctyl isothiocyanates from watercress are potent inducers of phase II enzymes. Carcinogenesis 2000; 21: 1983-8.
32. International Agency for Research on Cancer. Cruciferous Vegetables, Isothiocyanates and Indoles (Vol 9). IARC Handbook of Cancer Prevention, 2004. In: http://www.iarc.fr/en/publications/pdfs-online/prev/handbook9/index.php; accessed June 2013.
33. National Cancer Institute. Cruciferous vegetables and cancer prevention. In: https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/cruciferous-vegetables-fact-sheet; accessed July 2015.
– – – –
The complaint, therefore, that all topics are preoccupied, is nothing more than the murmur of ignorance or idleness, by which some discourage others, and some themselves; the mutability of mankind will always furnish writers with new images, and the luxuriance of fancy may always embellish them with new decorations.
La queja, por lo tanto, que todos los tópicos ya han sido tratados, es nada más que un murmullo de ignorancia o negligencia, por el cual algunos desaniman a otros, y algunos a sí mismos; la mutabilidad humana siempre proveerá a los escritores con nuevas imágenes, y la lujuriosa fantasía puede siempre embellecerlas con nuevas decoraciones.
Samuel Johnson (1709-1784)
Adventurer #95 (October 2, 1753). En: http://www.samueljohnson.com/writing.html#822;
consultado el 24/5/2017
